Saturated fat facts for kids
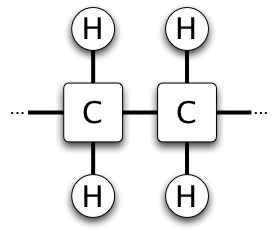
Saturated fat is a type of fat found in many foods. It's called "saturated" because its carbon atoms are completely full, or "saturated," with hydrogen atoms. This means there are no double bonds between the carbon atoms in its structure. Saturated fats are usually solid when they are at room temperature. Think of butter or the fat on a piece of meat.
For a long time, many scientists and doctors believed that eating a lot of saturated fat was a main cause of heart disease. However, new research has made this topic more complicated. Some recent studies suggest that the link between saturated fat and heart disease might not be as strong as once thought. This is still a topic that scientists are discussing and researching.
Where to Find Saturated Fat
Saturated fats are found in many foods we eat every day. Some common sources include:
- Butter and cheese
- Red meat, like beef and lamb
- Poultry skin
- Chocolate
- Some nuts and seeds
- Coconut oil and palm oil
The Science of Saturated Fat
When we say a fat is "saturated," it means its chemical structure is full of hydrogen atoms. Imagine a chain of carbon atoms. In a saturated fat, each carbon atom in the chain is connected to as many hydrogen atoms as it can hold. This makes the fat molecules very straight and able to pack together tightly, which is why they are solid at room temperature.
In contrast, unsaturated fat molecules have one or more double bonds between their carbon atoms. These double bonds create "kinks" or bends in the molecule, which stops them from packing together as tightly. This is why unsaturated fats, like olive oil, are usually liquid at room temperature.
Related pages
See also
 In Spanish: Grasas saturadas para niños
In Spanish: Grasas saturadas para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |