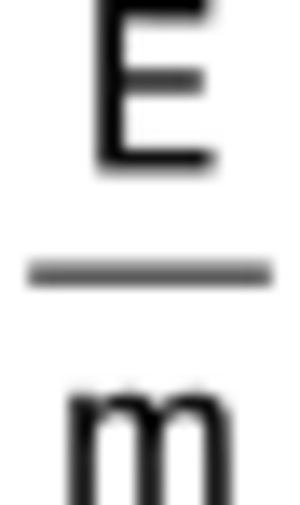Thermodynamic potential facts for kids
Thermodynamic potentials are like special measurements that tell us how much energy a system has and how it might change. Imagine a bouncy ball held high up – it has "potential energy" because it can fall and bounce. In a similar way, thermodynamic potentials describe the "potential energy" stored in things like gases, liquids, or even living cells.
These potentials help scientists understand how different systems behave when conditions change, like when they get hotter or colder, or when pressure is applied. They are super useful in fields like chemistry, physics, and engineering to predict what will happen in reactions or processes.
Contents
What Are Thermodynamic Potentials?
In the world of thermodynamics, which is the study of heat and energy, thermodynamic potentials are special numbers that describe the energy of a system. They are called "potentials" because they show the amount of energy a system has that could be used to do work, depending on what rules or "constraints" are put on it.
Think of it this way:
- If you have a balloon, its energy might change if you squeeze it (changing its volume).
- Its energy might also change if you heat it up (changing its temperature).
Different potentials help us understand these energy changes under different conditions.
Main Types of Thermodynamic Potentials
There are four main types of thermodynamic potentials that scientists often use:
Internal Energy (U)
The internal energy (written as U) is the total energy stored inside a system. This includes the energy from the movement of its tiny particles (like atoms and molecules) and the energy stored in the bonds between them. It's like the total energy account of everything inside a box.
When a system's internal energy changes, it usually means its temperature, pressure, or volume is also changing.
Helmholtz Free Energy (A)
The Helmholtz free energy (written as A or sometimes F) tells us how much useful work a system can do when its temperature and volume are kept constant. Imagine a chemical reaction happening in a sealed container that's kept at a steady temperature. The Helmholtz free energy helps predict if the reaction will happen on its own and how much energy it could release to do work.
It's calculated using the formula: 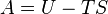 Where:
Where:
- U is the internal energy.
- T is the temperature.
- S is the entropy (a measure of disorder or randomness in the system).
Enthalpy (H)
Enthalpy (written as H) is very useful for understanding energy changes in processes that happen at constant pressure. Many chemical reactions, especially those in open containers, occur at constant atmospheric pressure.
Enthalpy helps us know how much heat is absorbed or released during these reactions. For example, when you burn a candle, the heat released is related to the change in enthalpy.
The formula for enthalpy is: 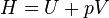 Where:
Where:
Gibbs Free Energy (G)
The Gibbs free energy (written as G) is perhaps the most commonly used potential in chemistry and biology. It helps predict whether a process or chemical reaction will happen on its own (spontaneously) when both the temperature and pressure are kept constant.
If the Gibbs free energy of a reaction goes down, it means the reaction is likely to happen by itself. This is super important for understanding everything from how batteries work to how our bodies digest food.
Its formula is: 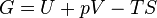 This combines parts of both Helmholtz free energy and enthalpy.
This combines parts of both Helmholtz free energy and enthalpy.
Why Are They Important?
Thermodynamic potentials are like different tools in a scientist's toolbox. Each one is designed to help understand energy changes under specific conditions. By using the right potential, scientists can:
- Predict if a chemical reaction will happen.
- Calculate how much energy can be extracted from a system.
- Design more efficient engines or power plants.
- Understand biological processes in living organisms.
They help us make sense of how energy moves and transforms in the world around us.
See also
 In Spanish: Potencial termodinámico para niños
In Spanish: Potencial termodinámico para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |