Ultraviolet–visible absorption facts for kids
Ultraviolet-visible absorption happens when a molecule soaks up special light. This light can be ultraviolet (like the light that causes sunburn) or visible light (the light we see). When a molecule absorbs this light, its tiny parts called electrons get excited. They jump from a calm, low-energy state to a higher, more energetic state.
Scientists use this idea in a method called ultraviolet-visible spectroscopy. It helps them learn about different substances.
Contents
How Molecules Absorb Light
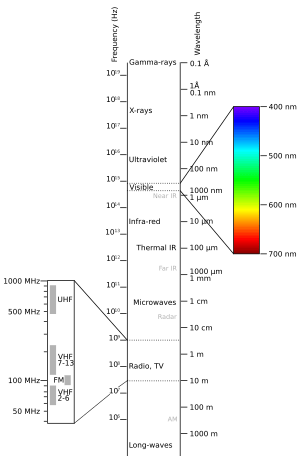
Different molecules absorb different types of light. For UV-visible absorption, the light wavelengths are usually between 200 and 800 nanometers. This range is a small part of the larger electromagnetic spectrum, which includes radio waves, X-rays, and more.
When light hits a molecule, its energy makes the outer electrons (called valence electrons) jump. They go from their normal, calm state to a higher energy state. A special machine called a UV-visible spectrophotometer measures this. It shows how much light a molecule absorbs at different wavelengths. This pattern is like a unique fingerprint for the molecule. It helps scientists understand what the molecule is made of and its properties. Not all molecules can absorb light in this specific way.
What Are Chromophores?
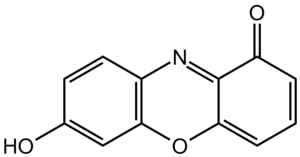
Molecules that absorb light in the UV-visible range are called chromophores. These are special parts of a molecule that are good at soaking up light. Chromophores often have "delocalized pi electrons."
Think of pi electrons as being in a special type of bond. When many of these bonds are in a molecule, the electrons can spread out. This spreading out (delocalization) helps the molecule absorb light. Many colorful substances, like dyes, have these delocalized pi electrons. Their color comes from how they absorb light.
For example, some molecules are used as pH indicators. These change color depending on whether a liquid is acidic or basic. Adding an acid or base changes how the pi electrons are spread out. This change makes the molecule show a different color.
Using Beer's Law to Find Concentration
The amount of light a molecule absorbs can tell us how much of that molecule is in a liquid. To figure this out, scientists use something called Beer's Law. It's a math equation that connects absorption to concentration.
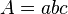
Scientists can find the values for Beer's Law in different ways. The absorption (A) is measured by the UV-visible spectrophotometer. The pathlength (b) is the thickness of the container holding the sample. This container, called a cuvette, is made of a material that doesn't absorb light itself. So, the light travels a known distance through the sample.
The "absorptivity coefficient" (a) tells us how strongly a molecule absorbs light at a certain wavelength. Scientists can calculate this by measuring a sample with a known concentration and pathlength. Once they know this constant, they can use Beer's Law to find the concentration of unknown samples.
Images for kids
See also
 In Spanish: Espectroscopia ultravioleta-visible para niños
In Spanish: Espectroscopia ultravioleta-visible para niños