West Nile virus facts for kids
Quick facts for kids West Nile virus |
|
|---|---|
 |
|
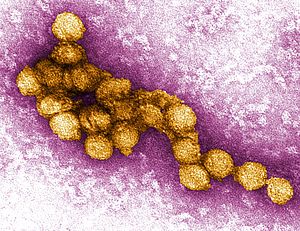
| A micrograph of the West Nile Virus, appearing in yellow | |
| Virus classification |
|
| (unranked): | Virus |
| Realm: | Riboviria |
| (unranked): | incertae sedis |
| Family: | Flaviviridae |
| Genus: | Flavivirus |
| Species: |
West Nile virus
|
The West Nile virus (WNV) is a tiny germ, also known as a virus, that causes an illness called West Nile fever. It belongs to a group of viruses called Flaviviridae, which also includes the Zika virus, dengue virus, and yellow fever virus.
Mosquitoes are the main way this virus spreads, especially certain types of Culex mosquitoes. The virus mostly lives in birds, moving from "bird–mosquito–bird" in a cycle. While birds are the main hosts, humans and horses can also get sick from the virus. Other animals rarely show symptoms.
Contents
Where the Name Comes From
The West Nile virus is not named directly after the famous Nile River. Instead, it got its name from the West Nile district of Uganda. This is where scientists first found the virus in 1937.
However, the names are connected in a way. The district in Uganda is named after the Albert Nile, which is a part of the Nile River in that area. After its discovery, the virus was found in many other parts of the world. It likely spread from this original West Nile district.
How the Virus Lives and Grows
Once the West Nile virus gets into an animal's body, it needs to enter the animal's cells to grow. The virus has special parts that help it stick to the surface of a cell. Think of it like a key fitting into a lock.
After attaching, the virus gets inside the cell by a process called endocytosis. This is like the cell swallowing the virus in a small bubble. Inside this bubble, the virus then releases its genetic material into the cell.
The cell then uses this genetic material to make more parts of the virus. These new parts come together to form new, complete viruses. These new viruses then leave the cell, ready to infect other cells or spread to new hosts.
Virus Family Tree
The West Nile virus is part of a group of viruses that includes the Japanese encephalitis virus and others. Scientists have studied the virus's history and believe it first appeared as a unique virus about 1,000 years ago.
Over time, this original virus split into two main types, called lineages. Lineage 1 is the one that has caused big outbreaks in Africa and around the world. Lineage 2 was mostly found in Africa. However, in 2008, Lineage 2 started appearing in horses in Europe.
The virus has spread widely since it was first found in North America in 1999. It has been reported across the United States, Canada, Mexico, and Central America. Many birds, humans, and horses have been infected. When many dead birds are found, especially crows, it can be an early sign that the virus is in the area.
Who Gets Infected and How it Spreads
The main animals that carry the West Nile virus are birds and mosquitoes. More than 300 different kinds of birds can get infected. Some birds, like American crows and blue jays, can die from the infection. Other birds, like American robins and house sparrows, are important carriers of the virus without always dying.
Many types of mosquitoes can carry the virus, but Culex species are the most important for spreading it. These mosquitoes often feed on birds.
The West Nile virus can also infect at least 30 types of mammals, including humans, horses, dogs, and cats. Some infected humans and horses can get sick, but dogs and cats rarely show symptoms. Reptiles and amphibians, like crocodiles, snakes, and frogs, can also get infected.
Mammals are usually "dead-end hosts." This means they don't get enough virus in their blood to infect another mosquito that bites them. So, the virus usually can't spread further from them.
In nature, the virus usually cycles between birds and mosquitoes. It can also spread between birds through direct contact or by eating infected birds. In cities, infected mosquitoes that have bitten birds can then bite humans, spreading the virus to people.
Rarely, the virus can spread through blood transfusions, organ transplants, or from a mother to her baby during pregnancy or breastfeeding. However, it does not usually spread directly from person to person.
The Illness
In Humans
Most people who get infected with West Nile virus do not feel sick at all. About 1 in 5 people who are infected will develop a fever and other symptoms like headache, body aches, joint pain, vomiting, diarrhea, or a rash.
A small number of people, less than 1 in 100, will develop a serious illness affecting the brain or spinal cord. This can include meningitis (swelling of the lining around the brain and spinal cord) or encephalitis (swelling of the brain). These severe cases can cause high fever, headache, neck stiffness, confusion, tremors, seizures, or even paralysis. Older people (over 60) and those with certain health conditions are more likely to get seriously ill.
In Horses
Horses can also get very sick from the West Nile virus. Luckily, there are now several vaccines available for horses. Before these vaccines, about 40% of infected horses in North America died.
How to Prevent Infection
Preventing West Nile virus infection mainly focuses on avoiding mosquito bites. This involves two main things:
- Protecting yourself: When you are in an area where WNV is present, try to avoid being outside when mosquitoes are most active (like at dawn and dusk). If you go outside, use an insect repellent that contains DEET. You can also wear clothing that covers more skin, such as long sleeves and pants.
- Controlling mosquitoes: Communities can work to control mosquitoes. This includes checking for and getting rid of standing water where mosquitoes lay their eggs. Examples of standing water are old tires, buckets, gutters, and swimming pools that are not being used. Reporting dead birds to local health departments can also help them track the virus.
Even though there are vaccines for horses, there isn't a vaccine for humans yet. The best way to lower your risk of getting infected is to avoid mosquito bites.
Climate Change and the Virus
Scientists are concerned that climate change might cause the West Nile virus to spread more. Changes in weather, like temperature, rainfall, and wind, can affect how many mosquitoes there are and where they live.
For example, warmer temperatures can make the virus grow faster inside mosquitoes. This can also lead to more mosquitoes in the summer. Heavy rainfall can also create more places for mosquitoes to lay eggs, which can increase their numbers. Wind can even help mosquitoes travel to new areas.
Mosquitoes can live in many different environments. However, changes in climate and how land is used can cause them to spread to new places or disappear from others. This can then affect human health.
See also
- West Nile fever