X-ray crystallography facts for kids
X-ray crystallography is a cool scientific method. It helps scientists see the exact three-dimensional (3D) shape of tiny things called molecules.
Imagine you want to know what a molecule looks like, but it's too small to see with a regular microscope. X-ray crystallography uses special light, called X-rays, to create a "picture" of the molecule. When X-rays hit the atoms in a molecule, they bend slightly. This bending creates a unique pattern that scientists can read to figure out the molecule's structure. This method works for many different types of molecules, from simple ones to very complex ones found in living things. The best part is that the sample isn't harmed during the process!
Contents
Who Discovered X-ray Crystallography?
The technique of X-ray crystallography was invented by a father and son team. Sir William Bragg (1862–1942) and his son, Sir Lawrence Bragg (1890–1971), worked together on this amazing discovery.
They won the Nobel Prize in Physics in 1915 for their work. Lawrence Bragg was the youngest person ever to receive a Nobel Prize. Later, he was the director at Cambridge University's Cavendish Laboratory. This was when the famous discovery of the structure of DNA was made in 1953 by James D. Watson, Francis Crick, Maurice Wilkins, and Rosalind Franklin.
How X-ray Crystallography Works
The oldest way to use X-ray crystallography is called X-ray diffraction (XRD). Here's how it generally works:
- First, scientists need a very pure, solid sample of the molecule they want to study. This sample must be in the form of a crystal.
- Next, they shine a beam of X-rays at this crystal.
- When the X-rays hit the atoms in the crystal, they scatter in different directions.
- This scattering creates a special pattern of spots on a detector screen.
- Scientists then analyze this pattern of spots. By looking at where the spots are and how bright they are, they can work backward to figure out how the atoms are arranged inside the crystal. This reveals the molecule's 3D shape.
What Can We Learn from X-ray Crystallography?
X-ray crystallography is a powerful tool used in many areas of science. It helps us understand the world at a very tiny level.
- Understanding Molecules: It shows us the exact arrangement of atoms in molecules. This is like having a blueprint for a tiny building.
- DNA Structure: This method was key to discovering the famous double-helix shape of DNA. Knowing DNA's structure helped us understand how life works.
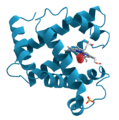
- Protein Shapes: Proteins are vital molecules in our bodies. X-ray crystallography helps scientists see the complex shapes of proteins. This knowledge is important for developing new medicines.
- New Materials: Scientists use it to design new materials with special properties, like stronger metals or new types of plastics.
Related pages
Images for kids
-
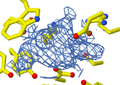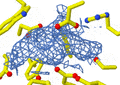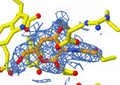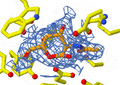
An X-ray diffraction pattern of a crystallized enzyme. The pattern of spots and their strength helps scientists figure out the enzyme's structure.
See also
 In Spanish: Cristalografía de rayos X para niños
In Spanish: Cristalografía de rayos X para niños
 | Leon Lynch |
 | Milton P. Webster |
 | Ferdinand Smith |