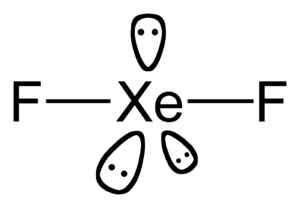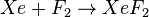Xenon difluoride facts for kids
Xenon difluoride is a special chemical compound. Its chemical formula is XeF2. This compound is made from a type of gas called a noble gas. It is very good at adding fluorine atoms to other chemicals. Xenon difluoride is the most stable of all known xenon compounds.
Contents
What is Xenon Difluoride?
Xenon difluoride is a compound that contains xenon and fluorine. It is a solid at room temperature. It has no color, so it looks white. It forms small crystals. In terms of its shape, the atoms in xenon difluoride line up in a straight, linear way.
How is it Made?
Scientists can make xenon difluoride. They do this by mixing xenon gas and fluorine gas together. This process needs high pressure and temperature. When these two gases react, they form solid xenon difluoride.
This equation shows that one atom of xenon (Xe) reacts with one molecule of fluorine (F2). This reaction creates one molecule of xenon difluoride (XeF2).
How is it Used?
Xenon difluoride is useful in organic chemistry. This is a branch of chemistry that studies compounds containing carbon. In organic chemistry, xenon difluoride is used as a "fluorinating agent." This means it helps to add fluorine atoms to other molecules. Adding fluorine can change how these molecules behave.
What Happens if You Heat It?
If you heat xenon difluoride, it can break apart. When it breaks down, it turns back into pure fluorine gas and xenon gas. This reaction can happen very quickly. It can even be explosive. This is because the solid compound turns into gases. Gases take up much more space than solids. This sudden increase in volume causes the explosive effect.
See also
 In Spanish: Difluoruro de xenón para niños
In Spanish: Difluoruro de xenón para niños
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |