Baking powder facts for kids
Baking powder is a special dry mix of chemicals. It helps make baked foods like cakes, cookies, and muffins light and fluffy. It works by creating tiny bubbles of carbon dioxide gas inside the dough or batter. This makes the food rise and become less dense. An English chemist named Alfred Bird invented baking powder in 1843.
It's often used when you don't want your food to taste like yeast. It's also great for mixtures that aren't stretchy enough to hold bubbles for a long time. Baking powder makes carbon dioxide much faster than yeast does. That's why breads made with it are called quick breads.
Contents
What is Baking Powder Made Of?
Most baking powders have three main parts:
- An alkaline ingredient, usually baking soda. This is where the carbon dioxide comes from.
- One or two acid salts. These acids react with the baking soda.
- A starch, like cornstarch or potato starch. The starch keeps the ingredients dry and stops them from reacting too soon. It also helps the powder flow easily.
How Does Baking Powder Work?
When baking powder gets wet, the acid and baking soda mix. This causes a reaction that releases carbon dioxide gas. Think of it like a mini science experiment happening right in your batter! The bubbles get trapped in the dough, making it expand and rise.
The chemical reaction looks like this:
- NaHCO3 + H+ → Na+ + CO2 + H2O
This means baking soda (NaHCO3) plus an acid (H+) creates a salt (Na+), carbon dioxide gas (CO2), and water (H2O).
History of Baking Powder
As mentioned, Alfred Bird invented baking powder in 1843. He was a chemist who wanted to create a leavening agent that didn't use yeast. Just two years later, in 1845, another inventor named Henry Jones created self-raising flour. This flour already had baking powder mixed into it, making baking even easier!
Images for kids
-
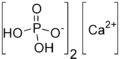
Monocalcium phosphate ("MCP") is a common acid component in domestic baking powders.
See also
 In Spanish: Polvo gasificante para niños
In Spanish: Polvo gasificante para niños