Energy level facts for kids
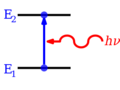
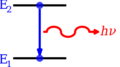
Think of energy levels as different "floors" or "steps" where tiny particles, especially electrons, can exist inside an atom or molecule. Just like you can't stand between steps on a staircase, electrons can only be on specific energy levels, not in between them. This idea comes from Quantum mechanics, which studies how very small things behave.
When an electron is "bound" to an atom, its energy level is usually a negative number. This is because we set the energy of an electron far away from the atom (at "infinity") as zero.
Sometimes, different ways an electron can be arranged might have the exact same energy level. When this happens, we call them degenerate energy levels.
This article will explain the main things that decide these energy levels in atoms and molecules.
Contents
Atoms and Their Energy Levels
Inside an atom, electrons move around the central nucleus. The main thing that sets an electron's energy level is how strongly it is pulled by the positive nucleus.
Electron Orbitals and Energy
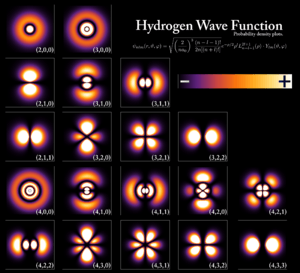
Each electron lives in a specific "orbital" around the nucleus. The energy of an electron in an orbital depends mostly on its distance from the nucleus and the charge of the nucleus.
Scientists use a number called the `principal quantum number` (often written as n) to describe these main energy levels. The lower the n value, the closer the electron is to the nucleus and the lower its energy.
Other Factors Affecting Energy Levels
While the main energy levels are set by the principal quantum number, other tiny effects can cause these levels to split into even finer steps.
- Fine Structure: This involves small changes due to how fast the electron moves and how its tiny internal "spin" interacts with the electric field of the nucleus.
- Hyperfine Structure: This is an even smaller effect, caused by the interaction between the electron's spin and the spin of the atom's nucleus.
- Electron Interactions: If an atom has more than one electron, these electrons push each other away. This pushing can slightly raise their energy levels. However, if the electrons are far apart, this effect is often very small.
How Outside Forces Change Energy Levels
Energy levels can also change if an atom is placed in an outside field.
- Zeeman Effect: If an atom is in a strong magnetic field, its energy levels can split or shift. This happens because electrons have a tiny magnetic property (like a tiny magnet) that reacts to the outside magnetic field.
- Stark Effect: Similarly, if an atom is in a strong electric field, its energy levels can also shift. This is because the electron's negative charge is pulled or pushed by the electric field.
Molecules and Their Energy Levels
Molecules are made of two or more atoms joined together. The energy of a molecule is a mix of several different types of energy:
- Electronic Energy: This is the energy of the electrons moving around the nuclei in the molecule.
- Vibrational Energy: This is the energy from the atoms in the molecule wiggling and vibrating.
- Rotational Energy: This is the energy from the whole molecule spinning around.
- Nuclear Energy: This is the energy stored within the nuclei of the atoms.
- Translational Energy: This is the energy of the whole molecule moving from one place to another.
Each of these types of energy can only exist at specific, "quantized" levels. The total energy of a molecule is the sum of all these different energy types. The exact energy levels depend on the specific molecule and its state.
Crystalline Materials
In solid materials like crystals, atoms are arranged in a very organized way. For these materials, important energy levels include:
- Valence Band: This is the highest energy level where electrons are usually found, holding the atoms together.
- Conduction Band: This is a higher energy level. Electrons need to jump to this band to move freely and conduct electricity.
- Fermi Energy: This is a special energy level that helps scientists understand how electrons fill up the available energy states in a material.
- Vacuum Level: This is the energy an electron needs to completely escape from the material into empty space.
- Defect States: Sometimes, tiny flaws or impurities in the crystal can create their own special energy levels.
See also
Images for kids
See also
 In Spanish: Nivel energético para niños
In Spanish: Nivel energético para niños
 | George Robert Carruthers |
 | Patricia Bath |
 | Jan Ernst Matzeliger |
 | Alexander Miles |