Osmosis facts for kids
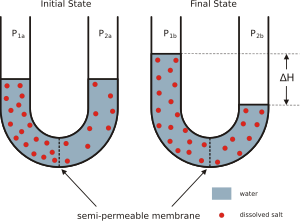
Osmosis is a special way that water (or other liquids) moves. It's the movement of solvent molecules, like water, through a thin barrier called a membrane. This movement happens from an area where there's a lot of water to an area where there's less water but more dissolved stuff (called solutes). It's like water trying to balance things out!
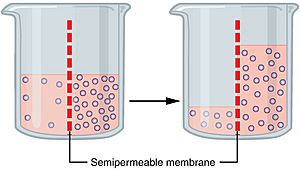
Imagine you have a glass with a special filter in the middle. On one side, you have pure water. On the other side, you have water with a lot of sugar dissolved in it. The filter (membrane) lets water molecules pass through, but it blocks the sugar molecules. What happens? Water molecules will naturally move from the pure water side to the sugary water side. They do this to try and make the amount of sugar more equal on both sides. This movement happens all by itself, without needing any extra push.
Osmosis is super important for living things, like plants, animals, and even you! This is because the membranes around our cells are like those special filters. They let water in and out, but they control what other things can pass through. This helps cells stay healthy and work properly.
Contents
What are Cell Membranes?
The outside layer of a cell is called the plasma membrane. Think of it like the cell's skin. This membrane is semi-permeable, which means it's picky about what it lets in and out. It allows small molecules, like water, to pass through easily. But it blocks larger molecules, like big proteins or sugars.
Sometimes, the membrane also has special "gateways" or "ports." These gates help bigger molecules get through. This process is called active transport. It uses energy and is very selective, meaning it only lets specific things pass. Cell membranes are mostly made of proteins and fats (lipids).
How Osmosis Works
Osmosis is when a liquid, usually water, moves across a special membrane. It moves towards the side that has more dissolved stuff (solute). Even though we often talk about water, osmosis can happen with other liquids and even gases!
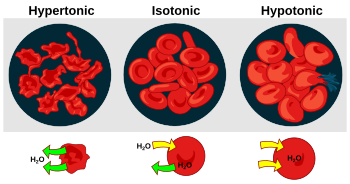
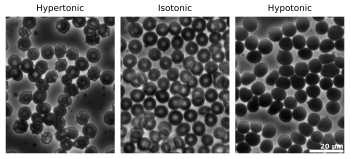
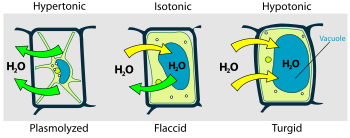
Let's think about a cell in water. If a cell is placed in pure water, water molecules will move from the outside (where there's lots of water) into the cell (where there's less water but more dissolved things inside the cell). This makes the cell swell up.
But if you put a cell in very salty water, the opposite happens. There's more water inside the cell than outside. So, water molecules will move out of the cell into the salty water. This makes the cell shrink.
You can see osmosis in action with a potato! If you put slices of potato into a very salty solution, the water from inside the potato will move out into the salt solution. This makes the potato slices shrink and become soft. The saltier the solution, the more water the potato loses and the smaller it gets.
Osmosis in Living Things
Osmosis is super important for how plants stay firm and upright. Water entering plant cells by osmosis pushes against their cell walls. This pressure is called turgor pressure. It helps plants stand tall and keeps their leaves from wilting.
- If a plant cell is in a very salty solution, water moves out of the cell. The cell becomes soft or flaccid. In extreme cases, the cell membrane might even pull away from the cell wall. This is called plasmolysis.
- If a plant cell is in pure water, water moves into the cell. The cell swells up and becomes firm or turgid. This is a healthy state for most plant cells.
Osmosis also helps plant roots soak up water from the soil. Plants use a process called active transport to gather dissolved nutrients in their root cells. This makes the water concentration inside the roots lower than in the soil. So, water naturally flows into the roots by osmosis. Osmosis also helps control how the tiny pores on leaves, called guard cells, open and close.
In humans, osmosis is vital for keeping our cells properly hydrated. It makes sure the right amount of water moves in and out of our cells. This balance of water and dissolved substances is key for our cells to work correctly. If this balance is off, cells can get sick or stop working.
Osmosis can also be dangerous in unusual environments. For example, if a freshwater fish is put into saltwater, or a saltwater fish is put into freshwater, they will quickly die. Their bodies can't handle the sudden change in how water moves in or out of their cells.
Related pages
Images for kids
See also
 In Spanish: Ósmosis para niños
In Spanish: Ósmosis para niños