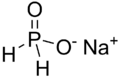Hypophosphite facts for kids
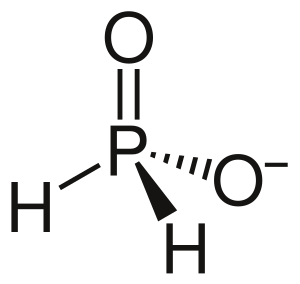
Hypophosphite is a special kind of tiny particle called an ion. Think of an ion as an atom or group of atoms that has an electric charge. The chemical formula for hypophosphite is H2PO2-. This formula tells us it's made of hydrogen (H), phosphorus (P), and oxygen (O) atoms, and it has a negative charge. In this ion, the phosphorus atom has a specific "oxidation state" of +1. This number helps chemists understand how atoms share or gain electrons in a chemical compound.
Hypophosphite is known for being a strong reducing agent. This means it can give away electrons to other substances in a chemical reaction. When it does this, it causes the other substance to change. Chemical compounds that contain these hypophosphite ions are simply called hypophosphites. It's important to know that hypophosphites are poisonous, so they must be handled carefully.
Contents
How Hypophosphite is Made
Hypophosphite is created through a process called disproportionation. This happens when phosphorus is placed in a warm alkali. An alkali is a type of chemical that is the opposite of an acid, like baking soda or soap. During this process, another chemical called phosphine is also produced. Hypophosphites are also known as salts of hypophosphorous acid. A salt is a chemical compound formed when an acid and a base react.
What are Hypophosphites Used For?
Hypophosphites are used in various ways, often because of their ability to act as reducing agents. For example, they can be used in the process of electroless nickel plating. This is a way to put a layer of nickel metal onto a surface without using electricity. This makes the surface stronger or more resistant to rust.
Related Pages
Images for kids
See also
 In Spanish: Fosfinato para niños
In Spanish: Fosfinato para niños