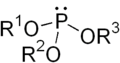Phosphite facts for kids
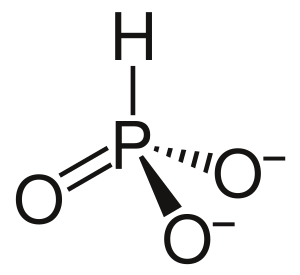
Phosphite is a special kind of ion. Think of an ion as a tiny particle that has an electric charge. The chemical formula for phosphite is HPO32-. This means it contains one hydrogen atom, one phosphorus atom, and three oxygen atoms, and it has a charge of 2- (meaning it has two extra electrons).
In the phosphite ion, the element phosphorus has a specific "power level" or oxidation state of +3.
Chemical compounds that contain the phosphite ion are called phosphites. These compounds are also known as the salts of phosphorous acid.
Phosphites can be poisonous, so they must be handled with care. They are also known as reducing agents. This means they can cause other chemical substances to gain electrons in a chemical reaction. An example of a phosphite compound is sodium phosphite.
Contents
What is Phosphite?
The phosphite ion is a small particle made of atoms. It carries an electric charge, which is why it's called an ion. Its specific formula, HPO32-, tells us exactly what atoms it's made of and its charge. The phosphorus atom inside the phosphite ion has a special chemical state, often called an oxidation state, of +3. This state describes how many electrons the phosphorus atom has gained or lost when it forms a chemical bond.
Phosphite Compounds
When the phosphite ion combines with other elements or groups of atoms, it forms new chemical substances called phosphites. These phosphite compounds are similar to what we call "salts" in chemistry. They are formed from phosphorous acid, much like table salt (sodium chloride) is formed from hydrochloric acid.
Properties of Phosphites
Phosphites have some important properties. One key thing to remember is that they can be poisonous. This means they are not safe to eat or touch without proper protection. Always be careful around chemicals!
Another important property is that phosphites are "reducing agents." In chemistry, a reducing agent is a substance that helps another substance gain electrons during a chemical reaction. It's like they "give away" electrons to other chemicals. This ability makes them useful in certain industrial processes. For example, sodium phosphite is a common phosphite compound used in various applications.
Related pages
Images for kids
-
The general structure of a phosphite ester showing the lone pairs on the P
See also
 In Spanish: Éster de fosfito para niños
In Spanish: Éster de fosfito para niños
 | Anna J. Cooper |
 | Mary McLeod Bethune |
 | Lillie Mae Bradford |