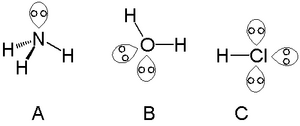
Lone pair facts for kids
Imagine tiny building blocks called atoms. These atoms connect to each other to form molecules, like water or air. They connect using special links called chemical bonds. A lone pair is a group of two electrons that belong to an atom but are not used to make a bond with another atom. Think of them as two electrons that are "unemployed" or "free" on an atom.
These lone pairs are always found in the outermost part of an atom, which is called the valence shell. This is the part of the atom where all the action happens, like forming bonds. Lone pairs, along with the electrons used in bonds, make up all the electrons in this outer shell. They usually have a lot of energy.
Lone pairs are very important for how molecules look and how they react with other molecules. They can even help form new connections between different molecules. For example, a type of molecule called a nucleophile uses its lone pair to "attack" and connect with another type of molecule called an electrophile. Lone pairs also take up more space around an atom than electrons that are in a bond. Because they are negatively charged, lone pairs on the same atom try to stay as far away from each other as possible. This push-and-pull helps decide the exact shape of a molecule.
Contents
What is a Lone Pair?
A lone pair is a pair of electrons that are not shared with another atom in a chemical bond. These electrons stay on one atom. They are found in the outermost layer of an atom, called the valence shell. This shell is where an atom's electrons are involved in bonding.
Why are Lone Pairs Important?
Lone pairs play a big role in how molecules are shaped. They also affect how molecules react with each other. Because lone pairs take up more space than bonding electrons, they push other parts of the molecule away. This pushing helps determine the molecule's overall shape.
How Lone Pairs Affect Molecular Shape
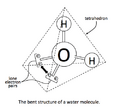
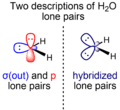
Think of a central atom with other atoms and lone pairs around it. The lone pairs push away from each other and from the bonding electrons. This pushing makes the molecule take on a specific 3D shape. For example, the water molecule (H₂O) has two lone pairs on its oxygen atom. These lone pairs push the hydrogen atoms closer together, giving water a bent shape instead of a straight line.
Lone Pairs in Chemical Reactions
Lone pairs are also very active in chemical reactions. They can be used to form new bonds with other atoms or molecules. Molecules that have lone pairs are often called nucleophiles. They can "donate" their lone pair to another molecule that needs electrons, which is called an electrophile. This is how many chemical reactions begin.
Images for kids
See also
 In Spanish: Par solitario para niños
In Spanish: Par solitario para niños
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |