Isolobal principle facts for kids
The isolobal principle (also called the isolobal analogy) is a smart way to guess how different parts of molecules will bond together. It's super useful in organometallic chemistry, which is a special type of chemistry that studies compounds with bonds between a metal and carbon atoms.
This principle helps connect the structure of organic molecules (like those found in living things) with inorganic molecular fragments (parts of molecules that don't have carbon-hydrogen bonds).
What is the Isolobal Principle?
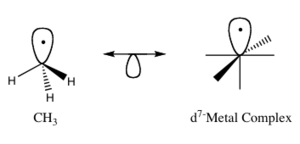
A famous scientist named Roald Hoffmann described molecular fragments as "isolobal" if they are similar in certain ways. This means they have a similar number of electrons, similar shapes, and similar energy levels in their "frontier orbitals."
Think of frontier orbitals as the outermost parts of a molecule where bonding happens. They include the highest occupied molecular orbital (HOMO), which is full of electrons, and the lowest unoccupied molecular orbital (LUMO), which is empty and ready to accept electrons.
If two different molecular fragments have similar frontier orbitals, we can predict how a less-known fragment will bond and react by looking at a better-known one. It's like finding a "twin" for a molecule part!
Isolobal compounds are similar to isoelectronic compounds. Isoelectronic compounds have the same number of valence electrons (electrons in the outermost shell) and a similar structure. The isolobal principle takes this idea a step further by looking at how the orbitals themselves are similar.
Who Discovered It?
Roald Hoffmann won the Nobel Prize in Chemistry in 1981 for his work on the isolobal analogy. He shared the prize with Kenichi Fukui. When he accepted his Nobel Prize, Hoffmann explained that the isolobal analogy is a very helpful, yet simple, idea. However, like many scientific models, it doesn't work perfectly in every single case.
Images for kids
See also
 In Spanish: Principio isolobal para niños
In Spanish: Principio isolobal para niños
 | George Robert Carruthers |
 | Patricia Bath |
 | Jan Ernst Matzeliger |
 | Alexander Miles |