Molecular vibration facts for kids
A molecular vibration is when the atoms in a molecule wiggle and jiggle around, but the whole molecule stays in the same spot. Think of it like a tiny dance the atoms do! These movements happen very, very fast. They are so quick that they happen trillions of times every second.
Molecules vibrate because their atoms are held together by bonds, like tiny springs. When a molecule absorbs energy, these springs can stretch, bend, or twist, making the atoms move. The amount of energy a molecule needs to start vibrating depends on how fast it wiggles.
For a simple molecule with just two atoms, like A−B, the atoms move back and forth, stretching and squeezing the bond between them. For molecules with many atoms, the vibrations are more complex. They can wiggle in different ways, called normal modes. Each normal mode is a specific way all the atoms move together at the same time.
When a molecule absorbs energy, it can start vibrating. This energy often comes from infrared light. Scientists use special tools like infrared spectroscopy and Raman spectroscopy to study these vibrations. By looking at how molecules vibrate, scientists can learn a lot about their shape and how their atoms are connected.
Contents
How Many Ways Can a Molecule Vibrate?
Every atom in a molecule can move in three directions (up/down, left/right, forward/backward). So, if a molecule has N atoms, it has 3N total ways it can move. These movements include:
- Moving as a whole: The entire molecule can move from one place to another. This is called translation. There are 3 ways for a molecule to translate.
- Spinning: The entire molecule can spin around. This is called rotation.
* For a molecule that is not straight (like a triangle or pyramid), there are 3 ways it can spin. * For a straight molecule (like a line), there are only 2 ways it can spin, because spinning along its own axis doesn't change anything.
To find out how many ways a molecule can vibrate, we subtract the ways it can translate and rotate from the total number of movements.
- For a molecule that is not straight, it has 3N – 6 vibrational modes.
- For a molecule that is straight (linear), it has 3N – 5 vibrational modes.
For example, a water molecule (H2O) has 3 atoms (N=3) and is not straight. So, it has (3 × 3) – 6 = 9 – 6 = 3 vibrational modes. This means a water molecule can wiggle in 3 different ways.
How Atoms Move in a Vibration
When a molecule vibrates, its atoms don't just move randomly. They move in specific patterns called internal coordinates. These describe how bonds stretch, or how angles between bonds change.
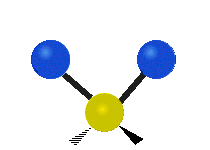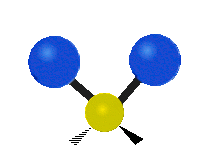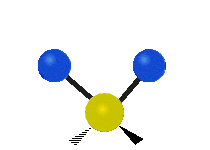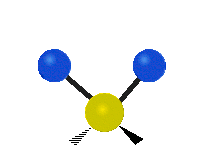
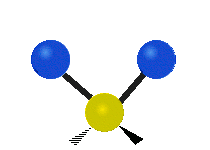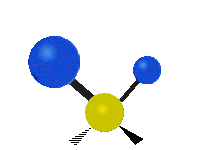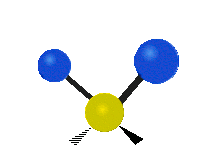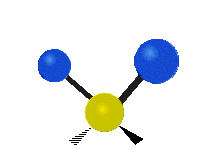
Here are some common ways atoms move in a vibration, using a group of atoms like –CH2– (a carbon atom with two hydrogen atoms attached) as an example:
- Stretching: The length of a bond changes.
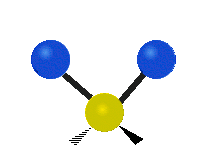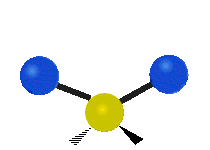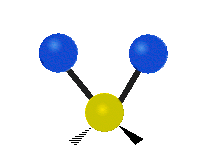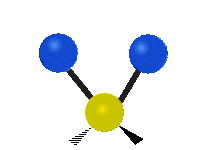
* Symmetrical stretching: Both hydrogen atoms move away from or towards the carbon atom at the same time. * Asymmetrical stretching: One hydrogen atom moves away while the other moves towards the carbon atom.
- Bending: The angle between two bonds changes.
* Scissoring: The two hydrogen atoms move towards and away from each other, like scissors closing and opening.
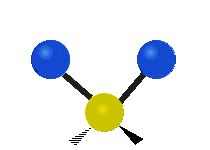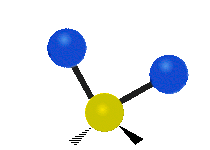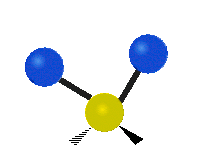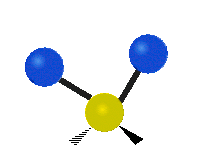
- Rocking: The whole group of atoms swings back and forth, like a rocking chair.
- Wagging: The group of atoms moves up and down out of its plane, like a dog wagging its tail.
- Twisting: One part of the molecule twists relative to another part.
These movements are often combined in the actual normal modes of vibration.
| Symmetrical stretching |
Asymmetrical stretching |
Scissoring (Bending) |
|---|---|---|
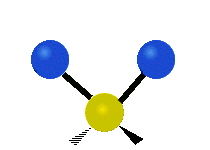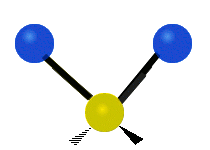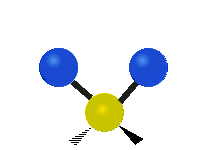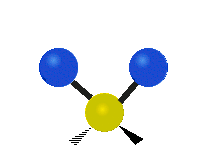 |
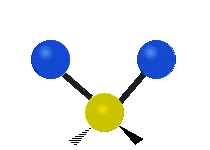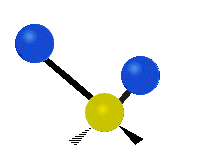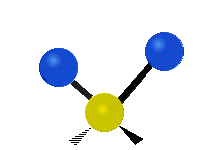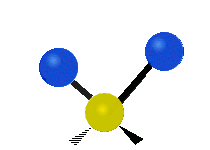 |
 |
| Rocking | Wagging | Twisting |
 |
 |
 |
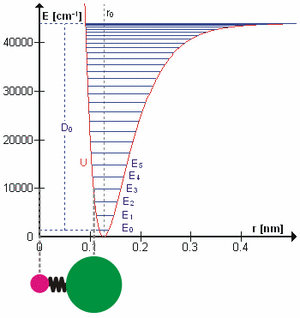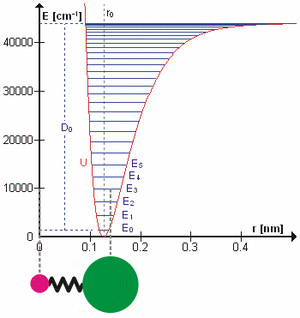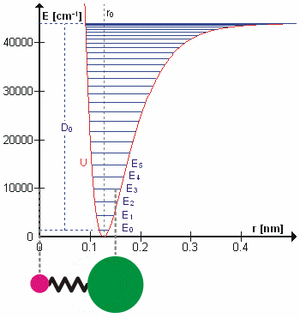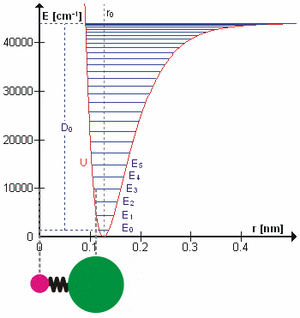
Vibrations and Energy
When a molecule vibrates, it has a certain amount of energy. This energy is not continuous; it comes in specific steps, like climbing stairs. Each step is called a "quantum" of energy. When a molecule absorbs exactly the right amount of energy, it jumps to a higher vibrational energy level, meaning it vibrates more strongly.
Scientists often use infrared spectroscopy to study these energy changes. Infrared light has just the right amount of energy to make molecules jump between vibrational levels. By shining infrared light on a sample and seeing which wavelengths are absorbed, scientists can figure out how the molecules are vibrating.
Sometimes, vibrations can also be studied using Raman spectroscopy, which uses visible light. These two methods are like two different ways to look at the same thing, and they often give scientists more complete information about a molecule's structure.
Images for kids
See also
 In Spanish: Vibración molecular para niños
In Spanish: Vibración molecular para niños
 | Aurelia Browder |
 | Nannie Helen Burroughs |
 | Michelle Alexander |