Radionuclide facts for kids
A radionuclide is a special kind of isotope that is radioactive. This means it gives off energy as it changes into a more stable form. Radionuclides can be found naturally, or they can be made by people in places like nuclear reactors. You might also hear them called radioactive isotopes or radioisotopes.
Contents
Radionuclides in Your Home
You might be surprised to learn that radionuclides are in many homes! They are used inside the most common household smoke detectors.
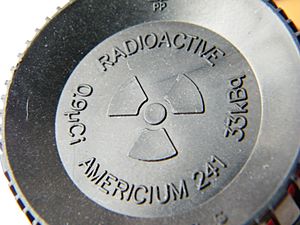
Americium-241 in Smoke Detectors
The radionuclide used in smoke detectors is called americium-241. Scientists create americium-241 by hitting plutonium with tiny particles called neutrons in a special machine called a nuclear reactor.
Americium-241 is radioactive, so it slowly changes over time. As it changes, it sends out tiny particles called alpha particles and a type of energy called gamma radiation. When it finishes changing, it becomes a different element called neptunium-237.
Smoke detectors use a very, very small amount of americium-241. It's usually less than one microgram, which is like a tiny speck of dust! This small amount is in the form of a compound called americium dioxide.
How Smoke Detectors Work
Americium-241 is perfect for smoke detectors because it sends out alpha particles. These alpha particles travel through the air inside a special part of the detector called an ionization chamber. As they move, they "ionize" the air, which means they give the air particles a small electric charge.
A small electric power is put across this charged air, which creates a tiny electric current. When smoke enters the detector, the smoke particles attach to the charged air particles. This makes the charged air particles heavier and slower, so they can't move as easily. This causes the electric current to drop. When the current drops enough, the smoke detector's alarm goes off, warning you about the smoke!
Images for kids
-
This image shows americium-241 giving off alpha particles inside a cloud chamber. You can see the paths of the particles!
See also
 In Spanish: Radioisótopo para niños
In Spanish: Radioisótopo para niños