Simple aromatic ring facts for kids
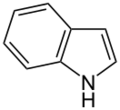
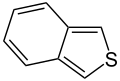
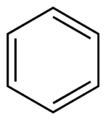
Simple aromatic rings, also called simple aromatics, are special kinds of organic compounds. Think of them as tiny, flat rings made of atoms, usually carbon. What makes them special is that they have a unique setup of electrons that move freely around the ring, making them very stable. You can find these simple rings as building blocks inside much larger and more complicated molecules. Some common examples are benzene, indole, and pyridine.
These simple aromatic rings can be made only of carbon atoms, or they can be heterocyclic. This means they might have other atoms in their ring besides carbon, like oxygen, nitrogen, or sulfur. They can also come in different sizes:
- Monocyclic means they have just one ring, like benzene.
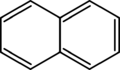
- Bicyclic means they have two rings joined together, like naphthalene.
- Polycyclic means they have many rings connected, like anthracene.
Often, simple rings have five or six atoms in their main ring. When rings are "fused" or "condensed," it means they share some of their connecting bonds.
Contents
What Makes a Ring Aromatic?
For a ring to be called "aromatic," it needs to follow a few important rules. These rules help explain why these molecules are so stable and special:
- It must be a ring: The atoms must be connected in a circle.
- It must be flat: All the atoms in the ring need to lie in the same flat plane.
- It must be fully connected: Every atom in the ring must have a special type of orbital (called a "p orbital") that can share electrons with its neighbors all the way around the ring. This creates a continuous path for electrons.
- It must have a special number of electrons: The ring needs to have a specific number of electrons moving freely around it. This number must fit a rule called Hückel's rule, which says it must be 2, 6, 10, 14, and so on (numbers that are 4 times any whole number plus 2).
If a molecule has 4 times any whole number of these special electrons (like 4, 8, 12), it's called "antiaromatic" and is usually very unstable.
Types of Aromatic Rings
Aromatic rings can be grouped based on the atoms they contain and how those atoms behave.
Rings with Nitrogen
Nitrogen (N) is a common atom found in heterocyclic aromatic rings. These rings can be divided into two main types:
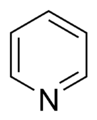
- Basic Aromatic Rings: In these rings, the nitrogen atom has a pair of electrons that are not part of the special electron cloud moving around the ring. These electrons are free to grab onto a hydrogen atom, making the molecule act like a "base" (which means it can accept a proton). A good example is pyridine. In these rings, the nitrogen atom is usually not connected to a hydrogen atom.
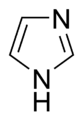
- Non-Basic Aromatic Rings: Here, the nitrogen atom's pair of electrons is part of the special electron cloud that moves around the ring. This means these electrons are busy making the ring aromatic and aren't free to grab a hydrogen atom, so the molecule is not basic. Examples include pyrrole and indole. In these rings, the nitrogen atom is usually connected to a hydrogen atom.
Some rings, like imidazole and purine, can even have both basic and non-basic nitrogen atoms in them!
Rings with Oxygen or Sulfur
In aromatic rings that contain oxygen or sulfur, these atoms also contribute to the special electron cloud. They have one pair of electrons that joins the aromatic system, while another pair of electrons stays outside the main ring system.
Images for kids
See also

- Polycyclic aromatic hydrocarbons (PAH)
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |