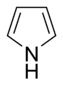Simple aromatic rings facts for kids
Simple aromatic rings, also called simple aromatics, are a special kind of organic compound. Imagine them as flat, ring-shaped molecules where electrons can move freely around the whole ring. This special movement of electrons makes them very stable. Many simple aromatic rings have common names you might hear, like benzene. They are often found as small parts of bigger, more complex molecules.
If a simple aromatic ring has atoms other than carbon in its ring, it's called "heterocyclic". These other atoms can be oxygen, nitrogen, or sulfur.
- Molecules with just one ring are called monocyclic, like benzene.
- Molecules with two rings are bicyclic, like naphthalene.
- Molecules with more than two rings are polycyclic, like anthracene.
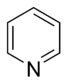
Common simple monocyclic aromatic rings often have five atoms in their ring, like pyrrole, or six atoms, like pyridine. When rings are "fused," it means they share some of their connecting bonds.
| Top - 0-9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z |
Meet Some Simple Aromatic Rings
Here's a look at some common simple aromatic rings. They are grouped by how many atoms are in their main ring and if they are fused (joined together).
|
||||||||||||||||||||||||||||||||||||||||||||||||||
Heterocyclic Aromatic Rings Explained
Heterocyclic aromatic rings are those that have atoms other than carbon in their ring structure. Nitrogen (N) is a common atom found in these rings.
- Basic Aromatic Rings: In these rings, the nitrogen atom has a pair of electrons that are not part of the special "aromatic" electron cloud. These electrons are free to grab onto a hydrogen atom, making the molecule act like a "base" in chemistry. An example is pyridine. Some rings, like imidazole and purine, can have both basic and non-basic nitrogen atoms.
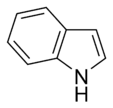
- Non-Basic Aromatic Rings: Here, the nitrogen atom's electron pair is part of the special electron cloud that makes the ring aromatic. Because these electrons are busy, the nitrogen atom usually has a hydrogen atom attached to it. Examples include pyrrole and indole.
For rings containing oxygen or sulfur, one of their electron pairs joins the aromatic system, similar to non-basic nitrogen rings. The other electron pair stays outside the system, like in basic nitrogen rings.
What Makes a Ring Aromatic?
To be called "aromatic," these molecules must follow a few important rules:
- The molecule must be shaped like a ring.
- Every atom in the ring must have a special type of orbital called a p orbital. These p orbitals must connect all the way around the ring, allowing electrons to move freely.
- The entire ring must be flat (planar).
- It must follow Hückel's rule. This rule says the ring needs a specific number of electrons in its special electron cloud: 2, 6, 10, 14, and so on. This can be written as (4n+2) pi electrons, where 'n' is any whole number starting from zero (0, 1, 2, 3...).
If a molecule has 4n pi electrons (like 4, 8, 12, etc.), it's actually considered "antiaromatic" and is usually less stable.
Related Topics
- Polycyclic aromatic compounds
- Polycyclic aromatic hydrocarbons (PAH)
See also
 In Spanish: Anillo aromático simple para niños
In Spanish: Anillo aromático simple para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |