Aluminium chloride facts for kids
Aluminium chloride (AlCl3) is a common chemical compound. It looks like a white or pale yellow solid. This solid is crystalline, meaning it has a regular, repeating pattern of atoms.
Aluminium chloride melts at a low temperature. It is made in different ways. One way is by mixing aluminium oxide with hydrochloric acid. If you want the form without water (called anhydrous), you can make it by reacting aluminium metal directly with chlorine gas.
This chemical is very useful! It's used a lot in making other chemicals. You might even find it in some deodorants because it helps stop sweating. It can be a bit irritating if it touches your skin or eyes, so it needs to be handled carefully.
Contents
What is Aluminium Chloride?
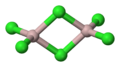
Aluminium chloride is a compound made of aluminium and chlorine. Its chemical formula is AlCl3. This means one aluminium atom is joined to three chlorine atoms.
Different Forms
Aluminium chloride can exist in different forms.
- The anhydrous form means it has no water. It's a white or pale yellow solid.
- The hexahydrate form means it has six water molecules attached to each aluminium chloride molecule. This form is also a solid.
How is it Made?
Aluminium chloride can be made in a few ways.
- One common way is to react aluminium oxide (which is found in bauxite ore) with hydrochloric acid. This creates aluminium chloride that has water in it.
- To get the anhydrous (water-free) form, you can react pure aluminium metal with chlorine gas. This reaction needs heat to happen.
What is it Used For?
Aluminium chloride has many important uses.
- Making Chemicals: It's a very important chemical in many industrial processes. It acts as a Lewis acid, which means it helps chemical reactions happen faster.
- Deodorants: A common use you might know is in antiperspirant deodorants. It helps to reduce sweat by blocking sweat ducts.
- Organic Chemistry: Scientists use it in labs for special reactions to create new organic compounds.
Safety Information
Aluminium chloride can be irritating.
- If it touches your skin or eyes, it can cause redness or discomfort.
- It's important to use gloves and eye protection when handling it.
- If you breathe in its dust, it can irritate your nose and throat.
Related pages
Images for kids
See also
 In Spanish: Cloruro de aluminio para niños
In Spanish: Cloruro de aluminio para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |