Chlordane facts for kids
Chlordane is a chemical that was used to kill insects, like a bug spray. It was first made in 1947. This chemical works by touching the insects and poisoning them.
Contents
What is Chlordane?
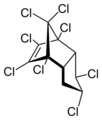
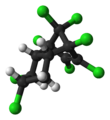
Chlordane is a type of chemical called an organochloride. It was created in a lab using a special chemical process. This chemical has many different parts, but they all work together to kill insects.
How it was Used
For many years, chlordane was very popular. It was used to protect crops like potatoes, wheat, and other vegetables by treating their seeds. This helped stop insects from eating the young plants.
People also used chlordane to protect wood from damage. It was very good at stopping pests like ants and termites from destroying homes and other wooden structures.
Why it's Dangerous
One big problem with chlordane is that it breaks down very slowly. This means it stays in the environment for a long time. It can easily spread far away from where it was first used.
Chlordane can also build up inside the bodies of animals. In animals like mammals (which include humans), it mostly collects in the nervous system and the liver.
Scientists have studied chlordane and found that it can cause cancer in mice. This means it might also cause cancer in humans. Chlordane is known to be toxic, which means it's harmful to living things.
Banned Worldwide
Because chlordane is so dangerous and stays in the environment for a long time, it is now banned in many countries. It is listed under an important international agreement called the Stockholm Convention on Persistent Organic Pollutants. This agreement helps to stop the use of chemicals that harm people and the planet.
See also
- Clordano para niños (In Spanish)
Images for kids
 | George Robert Carruthers |
 | Patricia Bath |
 | Jan Ernst Matzeliger |
 | Alexander Miles |