Conrotatory and disrotatory facts for kids
These terms describe two special ways that parts of a molecule can move during a type of chemical reaction called an electrocyclic reaction. Imagine a molecule that forms a ring, or a ring that opens up. The way the atoms at the ends of the changing part move is very important.
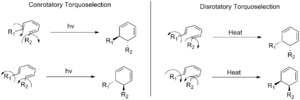
In a conrotatory movement, the parts at the ends of a double bond system move in the same direction. This means they both spin either clockwise or counterclockwise. Think of two gears turning together.
In a disrotatory movement, these parts move in opposite directions. One might spin clockwise while the other spins counterclockwise. Think of two gears turning away from each other.
These specific movements help new chemical bonds form correctly when a ring closes, or old bonds break correctly when a ring opens.
Contents
How Molecules Move: An Example
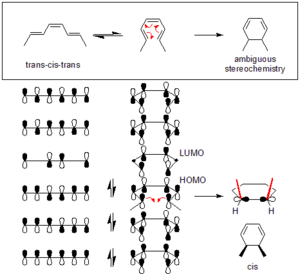
Let's look at an example. A molecule called octatriene can change into another molecule called dimethylcyclohexadiene. For this to happen, the parts of the octatriene molecule need to move in a disrotatory way. This specific movement helps the molecule form a new sigma bond (a strong single bond) with the right shape.
Heat, Light, and Molecular Movement
The way a molecule moves during these reactions depends on whether you add heat or light to make the reaction happen.
- When you use heat (called a thermal reaction), molecules with a certain number of pi electrons (specifically, 4n + 2 pi electrons, where 'n' is any whole number) will move in a disrotatory way.
- However, if you use light (called a photoinduced reaction) for these same molecules (4n + 2 pi electrons), they will move in a conrotatory way instead.
It's the opposite for molecules with 4n pi electrons (where the number of electrons can be divided by 4):
- With heat, these molecules move in a conrotatory way.
- With light, they move in a disrotatory way.
These rules are very important because they explain why some reactions happen in a specific way and create specific products.
Woodward–Hoffmann Rules
All these different ways molecules move during ring-opening or ring-closing reactions are summarized by a set of important guidelines called the Woodward–Hoffmann rules. These rules help scientists predict how these reactions will happen.
See also
 In Spanish: Conrotatorio y disrotatorio para niños
In Spanish: Conrotatorio y disrotatorio para niños