Crystallization facts for kids
Crystallization is a cool process where atoms or molecules come together in a very organized way. They form solid shapes called crystals. Think of it like building blocks neatly stacking up!
This process helps separate a solid material from a liquid or sometimes even a gas. Crystallization can happen naturally, like when snowflakes form. It can also be done by people in labs or factories.
When crystals grow quickly, they usually end up being small. An example of this is the rock basalt. If crystals grow slowly, they can become much larger, like the crystals you see in granite.
Contents
How We Make Crystals (Artificial Crystallization)
Sometimes, we want to make crystals on purpose. This is called artificial crystallization. It's a way to create solid crystals from a solution. A solution is a mixture where one substance is completely dissolved in another, like sugar in water.
For crystals to form, the solution needs to be "supersaturated." This means it has more dissolved stuff (called the solute) than it normally could hold. Imagine trying to dissolve so much sugar in water that it just can't take any more!
There are a few ways to make a solution supersaturated:
- Evaporation: Letting the liquid (solvent) dry up.
- Cooling: Making the solution colder.
- Chemical Reactions: Mixing chemicals that make the dissolved stuff less soluble.
- 'Drowning': Adding another liquid that makes the dissolved stuff less soluble.
A Simple Example: Sugar Crystals
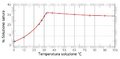
Let's use a simple example to understand supersaturation. 1. Imagine you have a glass of water. 2. You start adding sugar to it, stirring it in. 3. You keep adding sugar until no more will dissolve. This is a "saturated" solution. 4. Now, if you heat this sugar solution, you can actually dissolve even more sugar! This is because most solids dissolve better in warmer liquids. 5. When you've dissolved extra sugar by heating, you now have a "supersaturated" solution. It's holding more sugar than it normally would at room temperature. 6. As the hot solution cools down, the water can't hold all that extra sugar anymore. The "extra" sugar then starts to come out of the solution and form tiny sugar crystals. This is crystallization!
How Crystals Start and Grow
Crystallization happens in stages:
- Primary Nucleation: This is when the very first tiny crystal forms. It's like the seed for all the other crystals.
- Secondary Nucleation: Once you have a few tiny crystals (called "nuclei"), they help more crystals grow. These existing crystals act as guides, making it easier for new molecules to join and build bigger crystals. This stage is super important for making lots of crystals, like in a factory.
In our sugar example, those first few sugar crystals that appeared when the solution cooled were the nuclei. They then helped more sugar molecules join them, leading to more crystals forming.
Images for kids
-
Crystallized honey
-
Low-temperature SEM magnification series for a snow crystal. The crystals are captured, stored, and sputter-coated with platinum at cryo-temperatures for imaging.
-
Crystallization of sodium acetate.
See also
 In Spanish: Cristalización para niños
In Spanish: Cristalización para niños