Döbereiner's lamp facts for kids
The Döbereiner's lamp was one of the very first lighters ever invented! Imagine a time before matches or modern lighters. This clever device was created in 1823 by a German scientist named Johann Wolfgang Döbereiner. People used these lamps for many years, right up until about 1880. It was a truly revolutionary invention for its time.
Contents
What is Döbereiner's Lamp?
This special lamp isn't like the electric lamps we use today. It was designed to create a flame instantly. It worked by using a smart chemical trick. Instead of a spark, it used a reaction between two gases to make fire. This made it a very useful tool for starting fires or lighting candles.
How Does This Amazing Lamp Work?
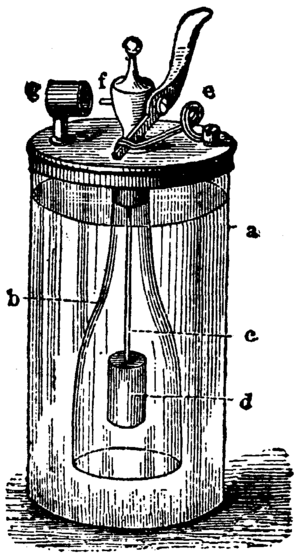
The Döbereiner's lamp works because of a cool chemical reaction. Inside the lamp, there's a glass cylinder (labeled a in the picture). This cylinder holds a strong liquid called sulfuric acid. There's also an open bottle (labeled b) inside, which contains pieces of zinc metal.
To make the lamp work, you open a small valve using a lever (labeled f). This allows the sulfuric acid to flow down into the bottle. When the acid touches the zinc, a chemical reaction begins! This reaction creates a gas called hydrogen.
The hydrogen gas then rises up and out of the bottle. It passes by a tiny piece of platinum (labeled g). Platinum is a special metal that acts as a "catalyst." This means it helps a chemical reaction happen faster without being used up itself.
The Secret of the Spark: Platinum!
When the hydrogen gas touches the platinum, something amazing happens. The platinum helps the hydrogen gas mix with oxygen from the air. This mixture of hydrogen and oxygen is called oxyhydrogen. When these two gases react on the platinum, they get very hot, very quickly. This sudden heat causes the gas mixture to burst into flame! It's like magic, but it's all science. This reaction also creates water vapor.
Stopping the Reaction
Stopping the flame is just as easy as starting it. You simply release the lever. This closes the valve. The hydrogen gas can no longer escape from the bottle. As more hydrogen gas is produced, it builds up pressure inside. This pressure pushes the sulfuric acid back up and away from the zinc. When the acid and zinc are separated, the reaction stops, and the flame goes out.
Where Can You See One Today?
Even though these lamps are very old, you can still see original Döbereiner lamps today! For example, the famous Heidelberg Castle in Germany has one. You can also find them at the Deutsches Museum (German Museum), which is a huge science and technology museum. It's a great way to see a piece of history and understand how early inventions worked.
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |