Diacetyl facts for kids
Quick facts for kids Diacetyl |
|
|---|---|
 |
|
 |
|
|
Preferred IUPAC name
Butane-2,3-dione
|
|
| Other names | Diacetyl Biacetyl Dimethyl diketone 2,3-Diketobutane |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C00741 |
| ChEBI | CHEBI:16583 |
| RTECS number | EK2625000 |
| SMILES | CC(=O)C(=O)C |
|
InChI
InChI=1/C4H6O2/c1-3(5)4(2)6/h1-2H3
|
|
| Beilstein Reference | 605398 |
| 3DMet | B00164 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Yellow liquid |
| Density | 0.990 g/mL at 15 °C |
| Melting point | |
| Boiling point | |
| 200 g/L (20 °C) | |
| Hazards | |
| Main hazards | Harmful, flammable |
| NFPA 704 |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
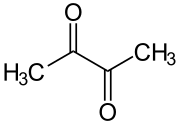
Diacetyl, also known as butanedione, is a special chemical compound. It is a yellow liquid with a very strong buttery smell and taste. Diacetyl is an organic compound and is found naturally in some alcoholic drinks. It is also added to certain foods to give them a buttery flavor.
Contents
How Diacetyl is Made
Diacetyl is created naturally during a process called fermentation. This is how many foods and drinks, like yogurt and beer, are made. In some bacteria, diacetyl forms when two smaller chemicals, pyruvate and acetyl CoA, combine.
Sour cream, buttermilk, and cultured butter get their tangy taste from special bacteria. These bacteria also produce diacetyl, which gives these foods their buttery smell and taste.
Industrially, diacetyl is made by changing another chemical called 2,3-butanediol.
Where Diacetyl is Used
In Food Products
Diacetyl is one of the main chemicals that gives butter its unique taste. Because of this, food makers often add diacetyl to products like margarine or artificial butter flavorings. This makes these products taste more like real butter.
In Electronic Cigarettes
Diacetyl is used as a flavoring in some liquids found in electronic cigarettes. People who use these e-cigarettes, and those around them, might breathe in diacetyl from the vapor.
In Alcoholic Drinks
Diacetyl is a byproduct of fermentation in alcoholic beverages.
Diacetyl in Beer
In some types of beer, like stouts or Scottish ales, a small amount of diacetyl is acceptable or even desired. It can add to the beer's flavor. However, in other beer styles, too much diacetyl is seen as a flaw.
During beer making, yeast produces diacetyl. Later, the yeast usually reabsorbs the diacetyl and changes it into other chemicals. Sometimes, brewers will slightly raise the beer's temperature after fermentation. This is called a "diacetyl rest." It helps the yeast absorb any remaining diacetyl.
Diacetyl in Wine
Some winemakers, especially those making chardonnay wine, purposely encourage diacetyl production. This gives the wine a certain feel and flavor, often described as "buttery."
Rules for Using Diacetyl
Food Regulations
The European Commission has approved diacetyl for use as a flavoring in all countries in the European Union. Scientists have studied diacetyl and found no safety concerns when it is used as a flavoring.
In 2007, a group of food safety experts in the EU began to evaluate diacetyl again.
In the United States, some companies that make butter-flavored popcorn started removing diacetyl from their products in 2007. This was a recommendation from a group of flavor manufacturers.
The U.S. Occupational Safety and Health Administration (OSHA) has also recommended safety steps. These steps help protect workers from breathing in too much diacetyl.
Electronic Cigarette Regulations
In 2016, diacetyl was banned from e-liquids and e-cigarettes in the European Union. This rule was part of the EU Tobacco Products Directive.
Images for kids
See also
 In Spanish: Diacetilo para niños
In Spanish: Diacetilo para niños



