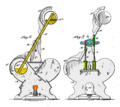Drinking bird facts for kids
Quick facts for kids Drinking bird |
|
|---|---|

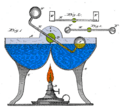
Drinking bird about to dip its beak in the water
|
|
| Classification | Heat engines |
| Application | Toy, Scientific demonstration |
| Fuel source | Heat transfer |
| Components | Bulbs, Tube, Axle, Support |
| Inventor | Miles V. Sullivan / Chinese Craftspeople |
| Invented | 1945 / much earlier than 1920 |
Drinking birds, also known as insatiable birdies or dipping birds, are fun toys that act like tiny heat engines. They look like a bird drinking water. People sometimes mistakenly think they are perpetual motion machines, but they are not! They need a temperature difference to keep moving.
Contents
What is a Drinking Bird Made Of?
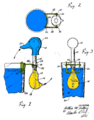
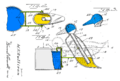
A drinking bird is usually made of two glass bulbs connected by a glass tube. This tube is the bird's "neck." The tube goes almost all the way into the bottom bulb. It connects to the top bulb but doesn't go inside it.
The space inside the bird is filled with a colorful liquid. This liquid is often dichloromethane. Older versions sometimes used other liquids.
The air is removed from inside the bird when it's made. This means the space is filled with vapor from the liquid. The top bulb has a "beak" and head covered in a soft, felt-like material. The bird often has paper eyes, a tiny hat, and tail feathers. The whole bird balances on a stand that lets it tip back and forth.
Be careful with drinking birds, as they are made of glass and can break. Some older versions contained liquids that could be harmful if the bird broke. Newer ones use safer liquids, but it's always best to handle them gently.
How Does a Drinking Bird Work?
A drinking bird is a heat engine. It uses a difference in temperature to create movement. It works in a cycle, like many engines.
Here's how the process happens:
- The bird's head is wet, usually from dipping into a glass of water.
- Water evaporates from the felt on the head. This is like sweat evaporating from your skin.
- When water evaporates, it cools the glass head.
- This cooling makes some of the vapor inside the head turn back into liquid (condense).
- The cooling and condensing cause the air pressure inside the head to drop.
- Meanwhile, the liquid in the bottom bulb is warmer. The higher pressure in the warmer bottom pushes the liquid up the neck tube.
- As the liquid rises, the bird becomes heavier at the top. It then tips forward, dipping its beak into the water.
- When the bird tips, the bottom end of the neck tube comes out of the liquid in the bottom bulb.
- A bubble of warm vapor from the bottom bulb can now rise up the tube. This lets the liquid flow back down to the bottom bulb.
- With the liquid back in the bottom, the bird becomes lighter at the top and swings back up to its starting position.
- The liquid in the bottom bulb then warms up again from the air around it.
If there's a glass of water for the beak to dip into, the bird will keep drinking and the cycle will continue! It will keep going as long as its head stays wet. The energy for this movement comes from the slight temperature difference between the bird's wet, cool head and its warmer body.
Science Behind the Sipping Bird
The drinking bird shows off several cool science ideas. That's why it's often used in chemistry and physics classes!
Here are some of the principles it demonstrates:
- Low Boiling Point Liquid: The liquid inside, like dichloromethane, boils at a low temperature. This means the bird can work even at room temperature.
- Gas Laws: The bird shows how temperature and pressure are related in a gas. When the head cools, the pressure drops.
- Evaporation and Condensation: It clearly shows how liquids turn into gas (evaporation) and gas turns back into liquid (condensation). These changes absorb or release heat.
- Torque and Center of Mass: The bird tips when its weight shifts, demonstrating torque (a twisting force) and how its center of mass changes.
- Capillary Action: The felt on the head soaks up water using capillary action, which is how liquids can move up narrow spaces.
- Wet-Bulb Temperature: The temperature difference between the head and body depends on how much moisture is in the air.
Another Type of Drinking Bird
In 2003, some scientists created a different kind of drinking bird. This one didn't use a special liquid inside or a temperature difference to move. Instead, it used capillary action and evaporation of water.
This bird works like this:
- It's balanced so that when it's dry, its head tips down.
- It's placed near water so its beak touches the water when it tips down.
- Water soaks up into the beak by capillary action. It then travels to a larger sponge area, like wings.
- When enough water is absorbed, the "wings" become heavy. This makes the bird tip back, lifting its head out of the water.
- With the beak out of the water, the water in the sponge slowly evaporates.
- When enough water has evaporated, the bird becomes lighter again. It then tips its head down to drink more water, and the cycle repeats.
This version moves much slower than the original drinking bird. It can take several hours for one full cycle!
Images for kids
See also
 In Spanish: Pájaro bebedor para niños
In Spanish: Pájaro bebedor para niños