Matter wave facts for kids
In quantum mechanics, which is a special part of physics, a matter wave is a way of thinking about matter (like everything around us) as if it were a wave. This idea was first suggested by a scientist named Louis de Broglie. It can be a bit tricky to imagine because we usually think of matter as solid things, like a ball or a table. But de Broglie's idea changed how scientists understood the tiny parts that make up everything.
Contents
What Are Matter Waves?
Imagine a stone you throw into a pond. It makes ripples, or waves, that spread out. We usually think of matter as tiny particles, like tiny little balls. But de Broglie proposed that matter, just like light, can act like both a particle and a wave. This idea is called wave-particle duality. It means that sometimes matter behaves like a solid particle, and other times it behaves like a wave.
De Broglie's Big Idea
De Broglie came up with a special equation to describe these matter waves. This equation helps us figure out the "wavelength" of any piece of matter. The wavelength is the distance between two peaks of a wave.
The equation looks like this: 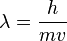
Let's break down what each part means:
- λ (lambda) is the wavelength of the matter.
- h is Planck's constant. This is a very tiny, fixed number that's important in quantum physics.
- m is the mass of the object (how much "stuff" it's made of).
- v is the velocity of the object (how fast it's moving).
There's another way to write this equation, which is: 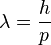
Here, p stands for momentum. Momentum is simply the mass of an object multiplied by its velocity (p = mv).
These equations show that if an object has a certain mass and is moving at a certain speed, it also has a wavelength, just like a light wave or a sound wave. For everyday objects, like a baseball or a car, their mass is so big that their wavelength is incredibly tiny – too small to notice. But for very tiny things, like electrons, their wave nature becomes very important!
Why Are Matter Waves Important?
The idea of matter waves helped scientists understand how tiny particles behave. For example, it helps explain how electrons move around the nucleus of an atom.
Later, another famous scientist named Erwin Schrödinger used de Broglie's ideas to create an even more advanced equation. This equation, called the Schrödinger equation, is a cornerstone of quantum mechanics and helps us predict how particles will behave.
Related Topics
- Quantum mechanics: The study of the smallest particles in the universe.
- Wave–particle duality: The idea that particles can act like waves, and waves can act like particles.
See also
 In Spanish: Ondas de materia para niños
In Spanish: Ondas de materia para niños
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |