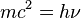Louis de Broglie facts for kids
Quick facts for kids
Louis de Broglie
|
|
|---|---|

Broglie in 1929
|
|
| Born | 15 August 1892 Dieppe, France
|
| Died | 19 March 1987 (aged 94) Louveciennes, France
|
| Alma mater | University of Paris (ΒΑ in History, 1910; BA in Sciences, 1913; PhD in physics, 1924) |
| Known for | Wave nature of electrons De Broglie–Bohm theory De Broglie waves |
| Awards | Nobel Prize in Physics (1929) Henri Poincaré Medal (1929) Albert I of Monaco Prize (1932) Max Planck Medal (1938) Kalinga Prize (1952) |
| Scientific career | |
| Fields | Physics |
| Institutions | University of Paris (Sorbonne) |
| Thesis | Recherches sur la théorie des quanta ("Research on Quantum Theory") (1924) |
| Doctoral advisor | Paul Langevin |
| Doctoral students | Cécile DeWitt-Morette Bernard d'Espagnat Jean-Pierre Vigier Alexandru Proca Marie-Antoinette Tonnelat |
Louis Victor Pierre Raymond, 7th Duc de Broglie (born August 15, 1892 – died March 19, 1987) was a French physicist. He came from a noble family. He made very important discoveries in quantum theory, which is a branch of physics that studies tiny particles.
In 1924, for his PhD, he suggested that electrons act like waves. He also thought that all matter, even big objects, has wave-like properties. This idea is called the de Broglie hypothesis. It shows that particles can sometimes act like waves, and waves can sometimes act like particles. This concept is a key part of quantum mechanics.
De Broglie won the Nobel Prize for Physics in 1929. This was after scientists proved his idea in 1927. They showed that matter really does behave like waves.
His ideas about particles acting like waves were used by Erwin Schrödinger. Schrödinger used them to create his famous wave mechanics. De Broglie was also a member of the Académie française and helped create CERN. CERN is a big European lab for nuclear research.
About Louis de Broglie
Early life and studies

Louis de Broglie was born in Dieppe, France. He was part of the famous de Broglie family. This family had many important military and political leaders in France for centuries.
Louis was the youngest of five children. His older brother, Maurice, also became a famous physicist. Louis grew up reading a lot. He loved history, especially politics. He had a great memory and could remember many details. People thought he would become a great politician.
He first studied history at university. But then he became interested in mathematics and physics. He earned a degree in physics.
Military service during World War I
When World War I started in 1914, Louis de Broglie joined the army. He worked in radio communications. He was sent to the Eiffel Tower, which had a radio transmitter.
He stayed in the military throughout the war. He worked on technical issues. For example, he helped set up wireless communication with submarines. He left the military in 1919. He later wished he had spent those six years on science.
His work in science
In 1924, Louis de Broglie wrote his PhD paper. It was called "Research on the Theory of the Quanta." In this paper, he introduced his theory of electron waves. This theory included the idea of wave–particle duality for matter. This means that matter can act like both a wave and a particle. He based his ideas on the work of Max Planck and Albert Einstein about light.
His research led to the de Broglie hypothesis. This idea states that any moving particle or object has a wave connected to it. De Broglie created a new area in physics called "wave mechanics." This field combines the physics of energy (waves) and matter (particles). For this amazing discovery, he won the Nobel Prize in Physics in 1929.
Later in his career, de Broglie worked on explaining wave mechanics. He wanted to show how it was based on cause and effect. This was different from the ideas that focused only on probabilities. His theory was improved by David Bohm in the 1950s. It is now known as the De Broglie–Bohm theory.
Besides his scientific work, de Broglie also wrote about the philosophy of science. He thought about the importance of new scientific discoveries. In 1930, he started a series of science books.
He became a member of the Académie des sciences in 1933. He was the academy's permanent secretary from 1942. In 1944, he was elected to the Académie Française. This is a very important French institution. His own brother, Maurice, welcomed him as a member. This was a unique event in the academy's history.
In 1952, UNESCO gave him the first Kalinga Prize. This was for his work in making science popular. In 1960, he became the 7th duc de Broglie. This happened after his older brother, Maurice, passed away without children.
Louis de Broglie never married. He passed away on March 19, 1987, at the age of 94.
Key Scientific Ideas
X-rays and the photoelectric effect
Louis de Broglie's first work was about x-rays and the photoelectric effect. He worked with his older brother, Maurice. They studied how X-rays are absorbed. They used Bohr's theory to explain this. They also looked at how X-rays affect photoelectron spectra. Their work helped scientists understand the inner parts of atoms.
Matter waves and duality
Louis de Broglie thought about the nature of X-ray radiation. He discussed it with his brother, who believed X-rays were a mix of waves and particles. This made Louis think about a theory that would connect particles and waves.
He started with Albert Einstein's idea of light quanta (tiny packets of light energy). In 1922, de Broglie suggested that light was made of these quanta. He then tried to connect this idea with how light shows interference and diffraction (wave-like behaviors). He concluded that light quanta must have some kind of wave connected to them.
In 1923, he made a big breakthrough. He suggested that any moving particle has an internal periodic process. This process has a frequency related to its energy. To make this work with Einstein's theory of relativity, de Broglie said that a "fictitious wave" travels with the moving body. This wave, later called a de Broglie wave, stays in phase with the particle's internal process.
He showed that if an electron moves in a closed orbit, this wave idea leads to the same rules that Bohr and Sommerfeld had found. This meant that the wave nature of particles could explain how atoms work.
This theory became the basis of wave mechanics. Albert Einstein supported it. It was proven by experiments like the electron diffraction experiments. Later, Erwin Schrödinger used and expanded on de Broglie's ideas.
De Broglie believed that a real wave was connected to particles. However, other physicists used a wavefunction (a mathematical tool) to describe matter's wave aspect. This wavefunction gives the appearance of wave behavior without a real physical wave. De Broglie spent the rest of his life trying to bring back the idea of real physical matter-waves. The de Broglie–Bohm theory is one way to give real meaning to these matter-waves.
Electron's internal clock
In his 1924 paper, de Broglie suggested that an electron has an "internal clock." He thought this clock was part of how a pilot wave guides a particle. Scientists are still trying to prove this idea. Recent experiments seem to support de Broglie's guess.
Mass of particles
De Broglie believed that neutrinos and photons (light particles) have a very tiny mass, not zero mass. He thought that if photons had no mass, his theory wouldn't make sense. Because of this, he also questioned the idea that the universe is expanding.
He also thought that the true mass of particles might not be constant. He believed it could change.
Principle of least action
In his 1924 paper, de Broglie connected two important principles in physics. One was the mechanical principle of least action. The other was Fermat's optical principle. He showed that the path a moving body takes is the same as the path a wave's light ray takes. This connection had been noticed by Hamilton a century earlier.
De Broglie always focused on the idea of "action" in physics. Max Planck had shown that "action" was a universal unit.
Duality in nature's laws
De Broglie saw a connection between continuous (like waves) and discontinuous (like particles) things. He thought that one could be a limit of the other. He always tried to combine different ideas in physics. For example, his first formula connects mechanics (mass) and optics (frequency):
Neutrino theory of light
In 1934, de Broglie suggested that a photon is like two Dirac neutrinos joined together. Most physicists today do not accept this theory.
Hidden thermodynamics
De Broglie's last idea was about the "hidden thermodynamics" of single particles. He tried to link three big ideas in physics: Fermat's principle, Maupertuis' principle, and Carnot's theorem (from thermodynamics).
In this idea, "action" becomes the opposite of "entropy" (a measure of disorder). He proposed an equation that links these two universal concepts. This theory suggests that the uncertainty principle is related to changes in entropy.
Honors and awards
- 1929 Nobel Prize in Physics
- 1929 Henri Poincaré Medal
- 1932 Albert I of Monaco Prize
- 1938 Max Planck Medal
- 1938 Fellow, Royal Swedish Academy of Sciences
- 1944 Fellow, Académie française
- 1952 Kalinga Prize
- 1953 Fellow, Royal Society
Images for kids
See also
 In Spanish: Louis-Victor de Broglie para niños
In Spanish: Louis-Victor de Broglie para niños