Photoelectric effect facts for kids
| Photoelectric effect | |
| Light-matter interaction | |
| Low energy phenomena | Photoelectric effect |
| Mid-energy phenomena | Compton scattering |
| High energy phenomena | Pair production |
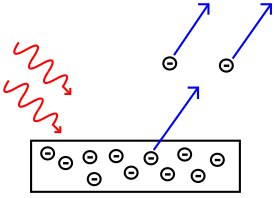
The photoelectric effect is a cool science idea in physics. It explains how light can make tiny particles called electrons jump off a metal surface. Imagine light as being made of tiny energy packets called photons. When a photon hits an electron on a metal, the electron can be kicked off. These electrons that get kicked off are called photoelectrons.
This effect is sometimes called the Hertz Effect. It was discovered by Heinrich Rudolf Hertz. However, the name "photoelectric effect" is used more often today. Understanding this effect helped scientists learn a lot about how light and electrons behave. It also led to the idea of wave–particle duality. This means light can act like both a wave and a particle. Albert Einstein explained the laws of the photoelectric effect. He won the Nobel Prize For Physics in 1921 for his work.
How the Photoelectric Effect Works
Not all light can cause the photoelectric effect. Only light with a certain amount of energy or more will work. This minimum energy is linked to the light's "cutoff frequency". Think of it as a minimum speed limit for the light. If the light's frequency is too low, nothing happens.
This "cutoff frequency" helps us find the work function. The work function is the amount of energy that holds an electron to the metal surface. It's like a sticky glue. Each metal has its own work function. It doesn't change based on the light hitting it.
If the light's frequency is higher than the cutoff frequency, electrons will jump off. These emitted electrons will also have some extra energy. This extra energy is called kinetic energy. It's the energy of movement.
The energy of a photon that causes the photoelectric effect follows a special rule. It's shown by the equation: 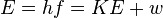 .
.
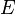 is the photon's energy.
is the photon's energy.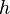 is Planck's constant. It's a very tiny number: 6.626×10−34 J·s.
is Planck's constant. It's a very tiny number: 6.626×10−34 J·s.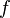 is the frequency of the light wave.
is the frequency of the light wave.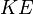 is the kinetic energy of the photoelectron.
is the kinetic energy of the photoelectron.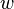 is the work function for the metal.
is the work function for the metal.
If a photon has a lot more energy, different things can happen. For example, Compton scattering or pair production might occur.
The brightness or intensity of the light itself doesn't make electrons jump off. The light must have the right frequency. But, if the frequency is high enough, making the light brighter will make more electrons jump off. It's like having more photons hitting the surface.
History of the Discovery
The first time someone noticed the photoelectric effect was in 1887. This was Heinrich Hertz. He saw that sparks jumped more easily between two charged metal spheres if light was shining on them. This was a big clue!
Later, in 1902, Philipp Lenard did more studies. He found that the energy of the kicked-off electrons didn't depend on how bright the light was. This was a puzzling discovery at the time.
It wasn't until 1905 that Albert Einstein fully explained the effect. He suggested that light acts like tiny particles (photons). These photons hit the electrons and make them jump off. This idea was quite new. Many scientists at the time thought light was only a wave.
In 1916, Robert Millikan did experiments using a special vacuum tube. His work showed that Einstein's equation for the photoelectric effect was very accurate. Even so, Millikan and others were slow to fully accept Einstein's idea of light particles.
The older idea that light was only a wave (called Maxwell's wave theory) couldn't explain the photoelectric effect. It also couldn't explain blackbody radiation. These mysteries were finally solved by a new field of physics called quantum mechanics.
Images for kids
See also
 In Spanish: Efecto fotoeléctrico para niños
In Spanish: Efecto fotoeléctrico para niños