Monsanto process facts for kids
The Monsanto process is a special way to make acetic acid. Acetic acid is the main ingredient in vinegar. This process uses a special helper chemical called a catalyst. The catalyst helps to combine two simpler chemicals: methanol and carbon monoxide.
This process works under high pressure (30 to 60 times the air pressure at sea level) and high temperature (150 to 200 degrees Celsius). It is very good at making almost pure acetic acid, with more than 99% of the product being what is wanted.
German chemists at BASF first developed this idea in 1960. Later, in 1966, chemists at the Monsanto company improved it with a new catalyst. Today, a similar but more modern process called the Cativa process is often used. It was developed by BP Chemicals Ltd and is cheaper and better for the environment. Because of this, the Monsanto process is not used as much in factories anymore.
How the Process Works (Catalytic Cycle)
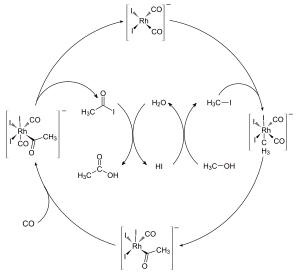
The Monsanto process works in a repeating cycle. This means the catalyst is used, helps make the product, and then is remade so it can be used again.
Here's a simpler look at the main steps:
- Starting the reaction: First, methanol is changed into a chemical called methyl iodide.
- Catalyst at work: The methyl iodide then joins with the special rhodium catalyst. This is a key step where the catalyst helps to add a carbon atom and an oxygen atom (a "carbonyl group") to the methyl iodide.
- Making the product: This new chemical then reacts with carbon monoxide. This forms another chemical called acetyl iodide.
- Getting acetic acid: Finally, the acetyl iodide reacts with water. This makes the final product, acetic acid, and also helps to get the catalyst ready to start the cycle all over again.
The most important step in this cycle is when the methyl iodide first joins with the rhodium catalyst. This step controls how fast the whole process goes.
Making Acetic Anhydride
The success of the Monsanto process also led to a way to make Acetic anhydride. This is another important chemical used in industry. It is made by reacting methyl acetate with carbon monoxide.
The process for making acetic anhydride is similar to the Monsanto process. It also uses special catalysts, like rhodium and lithium salts. However, acetic anhydride cannot be made with water present. So, this process must be done in very dry conditions. This is different from making acetic acid, which uses water in the final step.
See also
In Spanish: Proceso Monsanto para niños
 | Toni Morrison |
 | Barack Obama |
 | Martin Luther King Jr. |
 | Ralph Bunche |