Nuclear clock facts for kids
Quick facts for kids Nuclear Clock |
|
|---|---|
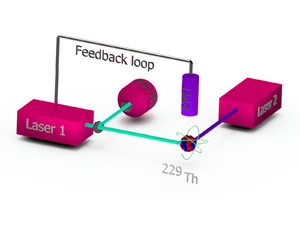
Concept of a thorium-229 based nuclear optical clock.
|
|
| Industry | scientific, satellite navigation, and data transfer |
| Application | time-keeping |
A nuclear clock is a super-accurate atomic clock that scientists are developing. Unlike regular atomic clocks that use energy changes in an atom's outer electrons, a nuclear clock uses a special energy change inside the atom's core, called the nuclear isomeric transition.
Scientists expect these clocks to be about 10 times more accurate than the best atomic clocks we have today. They could be so precise that they would only be off by about 1 second in 300 billion years!
The only atom core suitable for building a nuclear clock with today's technology is thorium-229m. This is a special version of thorium-229. It has the lowest known energy change in its nucleus. This energy change creates light in the vacuum ultraviolet region, which means we can use lasers to interact with it.
Contents
How Nuclear Clocks Work
Atomic clocks are the most accurate tools for keeping time. They work by using the fact that the energy difference between two electron states in an atom is always the same. When light hits an atom with exactly the right energy, it can make an electron jump to a higher energy level. This specific energy matches a particular frequency of light.
By shining light on many identical atoms and seeing how many get excited, scientists can fine-tune a light source to match this exact frequency. This frequency then becomes a super-stable reference for measuring time.
Early atomic clocks used microwave frequencies. But with the invention of lasers, we can now create very stable light frequencies. A tool called a frequency comb helps count these incredibly fast light waves (trillions of cycles per second) with amazing accuracy. Clocks that use lasers in this way are called optical atomic clocks.
For example, the ytterbium (Yb) lattice clock uses a specific electron change in the ytterbium-171 atom. In this clock, one second passes after the laser light has vibrated about 518 trillion times! Other super-accurate optical atomic clocks use strontium (Sr)-87 or aluminum (Al)-27. These clocks are accurate to about 1 second in 30 billion years, which is much longer than the age of the universe.
A nuclear optical clock works on the same idea. The big difference is that it uses an energy change in the atom's nucleus (its core) instead of its outer electrons. The nucleus is much smaller than the electron cloud, about 100,000 times smaller! This means the nucleus is much less affected by outside magnetic or electric fields. These outside forces are what limit how accurate electron-based atomic clocks can be. Because of this, nuclear clocks are expected to be even more accurate, possibly 10 times better than electron-based clocks.
Keeping Thorium Charged
An excited atom's nucleus can release its extra energy in two ways:
- By sending out a gamma ray (a type of light).
- By transferring the energy to an electron, which then gets ejected from the atom. This is called internal conversion.
For a nuclear clock to work best, scientists need to keep the thorium atoms in a charged state, called an ionized state. When thorium is ionized, it releases its energy as light (gamma rays) more slowly. This makes it easier to measure and use for a clock. Scientists can hold these charged atoms in special traps or embed them in crystals.
Types of Nuclear Clocks
Scientists are exploring two main types of nuclear optical clocks: trap-based nuclear clocks and solid-state nuclear clocks.
Trap-based Nuclear Clocks
In a trap-based nuclear clock, scientists trap either a single charged thorium-229 atom (called a single-ion nuclear clock) or a chain of several charged atoms (a multiple-ion nuclear clock). These clocks are expected to be the most accurate because the trapped atoms are mostly isolated from their surroundings. A multiple-ion clock might be even more stable than a single-ion clock.
Solid-state Nuclear Clocks
Since the nucleus is not much affected by the atom's outer electrons, scientists can also embed many thorium nuclei into a crystal. This is called a crystal-lattice nuclear clock. Because so many nuclei can be packed into a small space, this method could create a very strong signal. However, the nuclei in a crystal might be more affected by outside disturbances. Another idea is to shine light on a metallic thorium surface and detect the ejected electrons. This is known as an internal-conversion nuclear clock. Both solid-state ideas show promise for good performance.
What a Nuclear Clock Needs
For a nuclear clock to work, we need to be able to directly excite the nucleus with a laser. This is very hard for most nuclear changes because their energy levels are usually much too high for current laser technology.
Only two nuclear excited states are known to have low enough energy (below 100 eV):
- Thorium-229m, a special excited state of thorium-229, with an energy of about 8 eV.
- Uranium-235m1, an excited state of uranium-235, with an energy of 76.7 eV.
However, uranium-235m1 takes an incredibly long time to release its energy as light (trillions of years!), making it impractical for a clock. This leaves thorium-229m as the only realistic option for direct laser excitation.
Other important requirements for a nuclear clock are:
- The excited state of the nucleus must last long enough. A longer lifetime means a more precise measurement (a high quality factor).
- The basic thorium-229 atom must be easy to get and last a long time so scientists can work with it.
Fortunately, thorium-229m, when charged, has a good lifetime (around half an hour to decay to thorium-229). And thorium-229 itself lasts a very long time (about 7,917 years to decay to radium-225). These conditions make charged thorium-229m an excellent choice for building a nuclear clock.
History of Nuclear Clocks
The idea of using nuclear energy changes as a super-stable light source for measuring time was first suggested in 1996 by Eugene V. Tkalya.
Around 2000, the frequency comb was developed, which allowed scientists to measure optical frequencies very precisely. This led to the first proposal for a nuclear optical clock based on thorium-229m in 2003 by Ekkehard Peik and Christian Tamm. Their paper described both the single-ion and solid-state nuclear clock ideas.
Peik and Tamm suggested using individual, laser-cooled, charged thorium-229 atoms in a special trap. They proposed a method called the double-resonance method to detect if the nucleus had been successfully excited by a laser.
In 2012, Corey Campbell and his team further studied the expected performance of a single-ion nuclear clock. They predicted it could achieve an accuracy of about 1 second in 300 billion years, which would be much better than the best optical atomic clocks at the time.
In 2010, Eugene V. Tkalya also showed that thorium-229m could theoretically be used to create an ultraviolet laser.
The solid-state nuclear clock idea was further developed in 2010 by W.G. Rellergert and others. They predicted a long-term accuracy of about 1 second in 160 million years. While perhaps less accurate than the single-ion approach, solid-state clocks might be more compact and robust.
Important steps towards building a nuclear clock included successfully cooling charged thorium-229 atoms with lasers in 2011. In 2018, scientists detected a change in the atom's electron structure caused by the excited nucleus, which helped confirm the double-resonance method.
The exact energy of thorium-229m was hard to pin down for many years. Early measurements were incorrect, leading to many failed experiments. Scientists realized that the light from this energy change was easily blocked by air and common materials.
In 2016, scientists directly detected electrons released when the nucleus decayed. This helped determine the half-life of thorium-229m in neutral atoms in 2017 and led to the first laser-based study in 2018.
In 2019 and 2020, more precise measurements of the isomer's energy were made using different techniques.
Finally, in 2023, scientists clearly detected the photons (light particles) emitted by thorium-229m. In April 2024, two separate teams successfully excited the thorium-229m nucleus using tunable lasers. These breakthroughs meant the light frequency was known accurately enough to start building a prototype clock.
In September 2024, Jun Ye's laboratory at JILA made a direct comparison to a strontium-87 optical atomic clock. They measured the frequency of the thorium-229m transition very precisely, with a relative uncertainty of 1 part in a trillion. This measurement provided the exact frequency and energy needed for future nuclear clocks.
What Nuclear Clocks Can Do
Once nuclear optical clocks are fully operational, they will have many uses. Besides improving current applications like satellite navigation and data transfer, their extreme precision will open doors to new possibilities:
- Relativistic geodesy: This means super-accurate mapping of Earth's gravity and shape, which helps us understand our planet better.
- Searching for topological dark matter: These clocks could help scientists look for mysterious dark matter that we can't see directly.
- Checking if fundamental constants change: They can help determine if the basic rules of physics, like the fine-structure constant, change over time.
A nuclear clock is especially good at detecting tiny changes in the fine-structure constant. This is because the low energy of the thorium-229m transition results from a delicate balance of strong forces inside the nucleus. Even a tiny change in this constant would cause a proportionally huge change in the clock's frequency. Comparing a nuclear clock to a regular atomic clock could reveal if this fundamental constant is truly constant or if it changes over billions of years.
 | Ernest Everett Just |
 | Mary Jackson |
 | Emmett Chappelle |
 | Marie Maynard Daly |

