Chromatography facts for kids
Chromatography is a cool science trick used in labs to separate mixtures into their individual parts. Imagine you have a mixed bag of candies, and you want to separate them by color or type. Chromatography does something similar for tiny chemicals!
First, you dissolve the mixture in a liquid or gas. This is called the mobile phase because it moves. Then, this moving liquid or gas carries the mixture through a special system. This system has a material inside called the stationary phase, which stays still.
Different parts of your mixture like the stationary phase more or less. Because of this, they travel at different speeds through the system. This difference in speed makes them separate from each other. It's like a race where some runners get stuck in mud (the stationary phase) more than others, so they finish at different times!
Contents
History of Chromatography
Chromatography was first invented in 1900 by a Russian scientist named Mikhail Tsvet. He worked at the University of Kazan. Tsvet came up with the idea and the name "chromatography" in the early 1900s. He mainly used it to separate colors from plants, like the green in chlorophyll or the orange in carotenes.
Since these plant colors separated into different bands (like stripes) of green, orange, and yellow, they gave the technique its name. "Chroma" means color, and "graphy" means writing. So, it's like "color writing"!
In the 1930s and 1940s, new types of chromatography were developed. This made the technique useful for many more separation jobs.
The method really grew thanks to Archer John Porter Martin and Richard Laurence Millington Synge in the 1940s and 1950s. They even won the 1952 Nobel Prize in Chemistry for their work! They figured out the main ideas behind a type called partition chromatography. Their discoveries led to the fast growth of methods like paper chromatography, gas chromatography, and high-performance liquid chromatography. Since then, the technology has kept getting better and better. Scientists keep finding new ways to use chromatography to separate even very similar tiny molecules.
Key Chromatography Words
Here are some important words you might hear when talking about chromatography:
- Analyte – This is the substance you want to separate from a mixture. It's usually the part you are interested in.
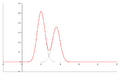
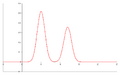
- Chromatogram – This is the picture or graph you get from a chromatography experiment. It shows the separated parts of your mixture as different peaks or patterns.
- Chromatograph – This is the actual machine or instrument that does the separation, like a gas chromatograph.
- Chromatography – This is the overall method of separating things. It uses two phases: one that moves (mobile phase) and one that stays still (stationary phase).
- Eluent – This is the liquid or gas that moves through the system. It's another name for the mobile phase.
- Eluate – This is the mixture that comes out of the separation system. It contains the separated parts and the mobile phase.
- Mobile phase – This is the part of the system that moves. It can be a liquid, a gas, or a special fluid. It carries your sample through the stationary phase.
- Preparative chromatography – This is when you use chromatography to get a lot of a substance. You want enough to use it for something else, not just to study it.
- Retention time – This is how long it takes for a specific substance to travel through the whole system. It's like the finish time in our race example.
- Sample – This is the mixture you are trying to separate. It can have one part or many.
- Solvent – This is any substance that can dissolve another substance. In liquid chromatography, it's usually the liquid mobile phase.
- Stationary phase – This is the material that stays still inside the system. It's fixed in place for the separation.
- Detector – This is the tool that finds and measures the separated substances after they come out of the system.
Chromatography works because different substances like to "partition" or divide themselves differently between the mobile and stationary phases. If the stationary phase is sticky for certain molecules, they will move slower. If it's not sticky, they will move faster.
Chromatography by Bed Shape
The "bed shape" refers to how the stationary phase is arranged.
Column Chromatography
In column chromatography, the stationary phase is packed inside a tube, like a tall, narrow column. The mixture is put at the top, and the mobile phase flows through it. Different parts of the mixture move at different speeds, so they come out of the column at different times.
In 1978, a faster version called flash column chromatography was invented. It uses pressure to push the solvent through the column quickly. This makes separations much faster, often less than 20 minutes! Modern flash systems use pre-packed tubes and can even be automated.
Planar Chromatography
Planar chromatography uses a flat surface for the stationary phase. This surface can be a piece of paper or a thin layer of special powder spread on a glass plate. The mixture travels different distances across this flat surface depending on how much it sticks to the stationary phase compared to how much it moves with the mobile phase.
Paper Chromatography
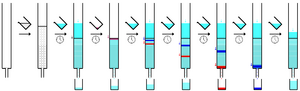
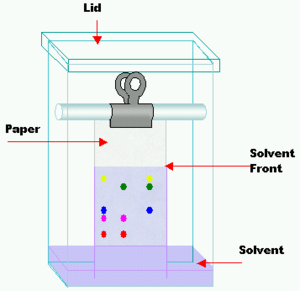
Paper chromatography is a simple technique. You put a small dot of your mixture onto a strip of special paper. Then, you place the paper in a container with a shallow layer of solvent and seal it. The solvent slowly climbs up the paper, like water soaking into a paper towel. As it moves up, it picks up the mixture.
The paper is made of cellulose, which is a "polar" substance (it has a slight electrical charge). Substances in your mixture that are less polar will travel further up the paper with the solvent. More polar substances will stick to the paper more strongly and won't travel as far. This separates them!
Thin-Layer Chromatography (TLC)
Thin-layer chromatography (TLC) is very similar to paper chromatography. But instead of paper, the stationary phase is a thin layer of a special powder, like silica gel, spread on a flat plate (often glass).
TLC is very useful because you can separate many samples at the same time on one plate. This makes it great for checking things quickly, like testing drug levels or how clean water is. It's also faster than paper chromatography and can give clearer separations.
Chromatography by Mobile Phase State
The mobile phase can be a gas, a liquid, or a special "supercritical fluid."
Gas Chromatography
Gas chromatography (GC) uses a gas as its mobile phase. The separation happens inside a long, thin tube called a column. These columns can be "packed" with a solid material or be very thin "capillary" tubes. Capillary columns are more expensive but give much better separations, especially for complex mixtures.
GC works by separating substances based on how they divide between a solid or thick liquid stationary phase and the moving gas (usually helium). The stationary phase is coated on the inside of the column. GC is used a lot in chemistry, like in the petrochemical industry (oil and gas), for checking the environment, and in chemical factories. However, it's not good for very large biological molecules like proteins because the high heat used in GC can damage them.
Liquid Chromatography
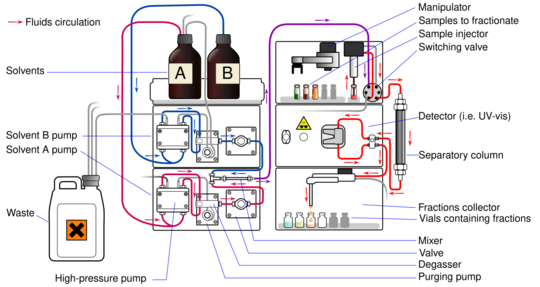
Liquid chromatography (LC) uses a liquid as its mobile phase. It can be done in columns or on flat plates. Today, a very common and powerful type is called high-performance liquid chromatography (HPLC).
In HPLC, the liquid mobile phase is pushed through a column at high pressure. The column is packed with tiny particles or a special porous material. HPLC is often divided into two types:
- Normal phase LC: The stationary phase is more polar (like silica), and the mobile phase is less polar (like toluene).
- Reversed phase LC: This is the opposite. The stationary phase is less polar (like C18), and the mobile phase is more polar (like a water-methanol mix). This is the most common type of HPLC.
Supercritical Fluid Chromatography
Supercritical fluid chromatography uses a special fluid as its mobile phase. This fluid is heated and pressurized so much that it acts like both a gas and a liquid at the same time.
Affinity Chromatography
Affinity chromatography is a very specific type of separation. It works because certain substances have a strong, natural "affinity" (liking) for specific molecules. Imagine a lock and key – only the right key (your substance) will fit into the lock (the stationary phase).
This method is often used in biochemistry to clean up proteins. Scientists can attach a special "tag" to a protein. This tag then sticks to the stationary phase. After the protein is purified, the tag can usually be removed, leaving just the pure protein.
Chromatography by Separation Mechanism
This describes how the separation happens.
Ion Exchange Chromatography
Ion exchange chromatography separates substances based on their electrical charge. It uses a stationary phase that has its own charge. This charged stationary phase attracts and holds onto substances with the opposite charge.
There are two main types:
- Cation-Exchange: The stationary phase has a negative charge, so it attracts positive ions (cations).
- Anion-Exchange: The stationary phase has a positive charge, so it attracts negative ions (anions).
This method is often used to purify proteins.
Size-Exclusion Chromatography
Size-exclusion chromatography (SEC) separates molecules based on their size. It's also called gel filtration chromatography. The stationary phase in SEC has tiny pores (holes) of a specific size.
Smaller molecules can get trapped inside these pores, so they take longer to travel through the column. Larger molecules are too big to enter the pores, so they just flow around them and come out first. This method is often used as a final step to make proteins very pure. It can also help scientists figure out the shape and structure of proteins.
Images for kids
See also
 In Spanish: Cromatografía para niños
In Spanish: Cromatografía para niños