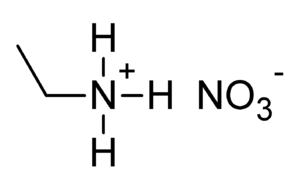Paul Walden facts for kids
Quick facts for kids
Paul Walden
|
|
|---|---|
Paul Walden
|
|
| Born | 26 July 1863 Rozulas, Governorate of Livonia, Russian Empire (now Pīpēni, Stalbe parish, Latvia)
|
| Died | 22 January 1957 (aged 93) Gammertingen, West Germany
|
| Nationality | German |
| Alma mater | Riga Technical University |
| Known for | Walden inversion Walden's rule Ethylammonium nitrate |
| Awards | Gmelin-Beilstein Medal (1954) |
| Scientific career | |
| Institutions | University of Rostock |
Paul Walden (Latvian: Pauls Valdens; Russian: Павел Иванович Вальден; German: Paul von Walden; 26 July 1863 – 22 January 1957) was an important chemist. He was from Latvia and Germany. He is famous for his work in stereochemistry. This is the study of how atoms are arranged in 3D space within molecules. He also studied the history of chemistry.
Walden invented a special chemical reaction called the Walden inversion. He also created the first ionic liquid that stays liquid at room temperature. This was called ethylammonium nitrate.
Contents
Early Life and School Days
Paul Walden was born in 1863 in a place called Rozulas, which is now in Latvia. He came from a large farming family. Sadly, he lost his father when he was four and his mother later on.
His two older brothers helped him financially. They lived in Riga, a big city. Thanks to their help, Paul could finish his schooling. He graduated with honors from a school in Cēsis in 1876. Then, he went to the Riga Technical High School and finished in 1882.
Starting Chemistry Studies
In December 1882, Paul started studying at the Riga Technical University. He quickly became very interested in chemistry. In 1886, he published his first scientific paper. It was about how to find nitric acid using color changes.
In 1887, he joined the Russian Physico-chemical Society. Around this time, he began working with Wilhelm Ostwald. Ostwald later won the Nobel Prize in Chemistry in 1909. This partnership really helped Paul grow as a scientist. Their first joint work was about how well salt solutions conduct electricity.
Discoveries in Chemistry
In 1888, Walden earned his degree in chemical engineering. He continued working at the Chemistry Department as an assistant. He worked with Professor C. Bischof.
Writing a Chemistry Handbook
Under Professor Bischof's guidance, Walden started writing a "Handbook of Stereochemistry." This book was published in 1894. To prepare for the handbook, Walden did many chemical experiments. He published 57 articles on stereochemistry between 1889 and 1900.
He also kept studying physical chemistry. In 1889, he found that how well a non-water solvent can ionize (break molecules into charged particles) depends on its dielectric constant.
Advanced Studies and Professorship
During his summer breaks in 1890 and 1891, Walden visited Ostwald at the University of Leipzig. In September 1891, he earned his master's degree there. He studied the "affinity values" of certain organic acids. Ostwald wanted him to stay in Leipzig, but Walden hoped for a better job in Riga.
In 1892, he became an assistant professor of physical chemistry. A year later, he earned his doctorate degree. His research was on osmotic phenomena. In September 1894, he became a full professor at Riga Technical University. He taught analytical and physical chemistry there until 1911. From 1902 to 1905, he was even the rector (head) of the university.
The Walden Inversion
In 1895, Walden made his most famous discovery. It was later called the Walden inversion. He found that you could get different forms of the same chemical compound. This happened through certain exchange reactions involving hydrogen. This discovery was a big part of his thesis in 1899.
After this, Walden became interested in electrochemistry. This is the study of how electricity and chemical reactions interact. He focused on solutions that don't use water. In 1902, he suggested a theory about how some solvents break down into ions by themselves.
In 1905, he found a link between how well a substance conducts electricity and how thick (viscous) it is. In 1906, he created the term "solvation". This describes how solvent molecules surround and interact with solute molecules. These discoveries, along with his work on stereochemistry, made him very well-known. He was even considered for the Nobel Prize in Chemistry in 1913 and 1914.
A Talented Teacher
Paul Walden was also known as a great chemistry lecturer. He wrote in his memories that his lectures were usually full. He said that the positive feedback from his students gave him energy. He liked to give his lectures spontaneously to keep them fresh. He never saw teaching as a burden.
In 1896, the Riga Technical University changed some rules. Before, all teaching was in German. Walden was the only professor who taught some courses in Russian. After the reforms, Russian became the official language. This change helped the university get money from the Russian government. It also helped students find jobs in Russia.
Walden worked with Ostwald again in an unusual way. Walden was rebuilding the Chemistry Department. Ostwald sent him the plans for the chemical labs in Leipzig as an example. In May 1910, Walden was chosen to be a member of the St. Petersburg Academy of Sciences. In 1911, he was invited to Saint Petersburg. He was asked to lead the Chemical Laboratories of the academy. These labs were started in 1748 by Mikhail Lomonosov.
Walden stayed in that position until 1919. He was allowed to live in Riga because he had better research tools there. But he traveled almost every week by train to St. Petersburg for meetings. Between 1911 and 1915, Walden published 14 articles. These were about the electrochemistry of non-water solutions. In 1914, he created the first ionic liquid that is liquid at room temperature. This was ethylammonium nitrate, which melts at 12 °C (53.6 °F).
After 1915, World War I and political problems in Russia made it hard for him to do research. He focused more on teaching and managing science projects. Because of political unrest in Latvia, Walden moved to Germany. He became a professor of inorganic chemistry at the University of Rostock. He worked there until he retired in 1934.
In 1924, he was invited back to Riga to give lectures. He was offered important chemistry jobs in Riga and St. Petersburg, but he said no. Even though he moved away, Walden remained popular in Russia. In 1927, he became a foreign member of the Russian Academy of Sciences. Later, he also joined the Swedish (1928) and Finnish (1932) Academies.
Family Life
Paul Walden's daughter was Antonina Anna Walden (1899–1983). She was a music teacher. She married a Finnish translator and writer named Juho August Hollo. Their son was the Finnish poet and translator Anselm Hollo.
Later Years and Legacy
In his later years, Walden focused on the history of chemistry. He collected a very special library with over 10,000 books. Sadly, his library and house were destroyed during a bombing in Rostock in 1942.
Walden then moved to Berlin and later to Frankfurt am Main. He became a visiting professor of the history of chemistry at the university there. He was in the French part of Germany when World War II ended. This meant he was cut off from Rostock University, which was in the Soviet part. So, he had no income.
German chemists helped him get a small pension. He gave occasional lectures and wrote his memories. In 1949, he published his most famous book, "History of Chemistry." He passed away in Gammertingen in 1957 at the age of 93. His memories were published later, in 1974.
See also
 In Spanish: Paul Walden para niños
In Spanish: Paul Walden para niños