Phosphonium facts for kids
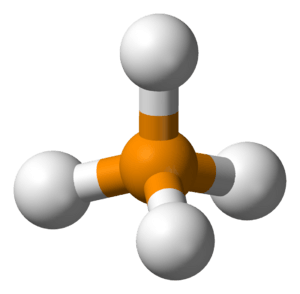
Phosphonium is a special kind of tiny particle called an ion. An ion is an atom or a group of atoms that has an electrical charge, either positive or negative. Phosphonium has a positive charge. It's not very common to find it by itself. Its chemical formula is PH4+, which means it's made of one phosphorus atom and four hydrogen atoms, and it carries a positive charge.
Phosphonium is quite similar to another common ion called ammonium, which has the formula NH4+. You'll mostly find phosphonium as part of larger, more complex substances, especially those known as organic compounds. Organic compounds are a huge group of chemicals that usually contain carbon and hydrogen. The weight of one phosphonium ion, called its molar mass, is about 35.01 grams for every unit of it.
Contents
How is Phosphonium Formed?
Phosphonium ions are usually made when a chemical called phosphine (PH3) gains an extra proton. A proton is a tiny, positively charged particle found in the center of atoms. When phosphine picks up a proton, it becomes PH4+, which is the phosphonium ion.
Where is Phosphonium Found?
You won't often find phosphonium ions floating around on their own. Instead, they are usually part of larger chemical structures. These structures are often organic compounds, which means they are based on carbon chains. For example, phosphonium can be found in compounds used to make certain types of plastics or in chemicals used in laboratories.
Phosphonium in Everyday Life
While you might not hear about phosphonium every day, some compounds containing it are used in important ways. For instance, some phosphonium compounds are used in the textile industry to make fabrics more resistant to fire. Others are used in chemical reactions to create new materials.
Images for kids
See also
 In Spanish: Fosfonio para niños
In Spanish: Fosfonio para niños
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |