Solvent extraction facts for kids
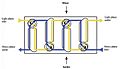
Solvent extraction, also known as Liquid–liquid extraction or partitioning, is a method to separate a compound based on the solubility of its parts. This is done using two liquids that don't mix, for example water and an organic solvent. Imagine you have a mixture, and you want to pull out just one part of it. This method uses two liquids that stay separate, like oil and water. The part you want to separate will dissolve in one liquid, leaving the other parts behind.
For example, if you have a substance like neutral Benzil (which is yellow) mixed with something else, and you add two liquids that don't mix, the Benzil will stay in the "organic" liquid. If the organic liquid is lighter than the water, it will float on top. This is how you can separate things based on how well they dissolve in different liquids.
Contents
How Does Solvent Extraction Work?
This process works because different substances dissolve better in different liquids. When you mix two liquids that don't blend, like oil and water, and add a third substance, that substance will choose to dissolve more in one of the liquids.
Think of it like this:
- You have a mixture of two things.
- You add two liquids that don't mix together.
- One part of your mixture will move into the first liquid.
- The other part will stay in the second liquid, or not dissolve at all.
- Because the two liquids stay separate, you can easily pour them apart, taking your separated substance with its chosen liquid.
Where Is Solvent Extraction Used?
Solvent extraction is a very useful method in many different areas. It helps us get important materials or clean up mixtures.
Everyday Products
This method is used to make many products you might use or see every day.
- Perfumes: It helps extract the fragrant oils from plants to create your favorite scents.
- Vegetable Oil: It's used to separate oils from seeds, like sunflower or soybean oil, that you use for cooking.
- Biodiesel: This fuel, made from plants or animal fats, also uses solvent extraction in its production.
Nuclear Fuel Recycling
A very important use of solvent extraction is in the nuclear industry.
- It helps recover plutonium from used nuclear fuel.
- This process is called nuclear reprocessing.
- The recovered plutonium can then be used again as new nuclear fuel. This helps save resources and reduce waste.
Images for kids
See also
 In Spanish: Extracción líquido-líquido para niños
In Spanish: Extracción líquido-líquido para niños
 | Jewel Prestage |
 | Ella Baker |
 | Fannie Lou Hamer |