Tin(II) sulfate facts for kids
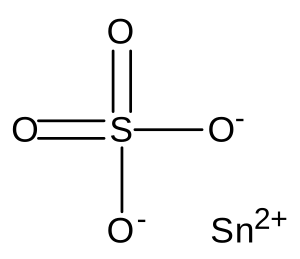
Tin(II) sulfate is a special kind of chemical compound. Think of a chemical compound as two or more different chemical elements joined together. Its chemical formula is SnSO4. This means it's made from one tin atom (Sn) and one sulfate group (SO4). The tin part in this compound has a specific electric charge, which scientists call its "+2 oxidation state".
Contents
What is Tin(II) Sulfate Like?
Tin(II) sulfate is a white, shiny solid. It looks a bit like tiny crystals, similar to sugar or salt. Unlike some other tin compounds, it doesn't act as a "reducing agent". This means it doesn't easily give away electrons to other chemicals.
One interesting thing about Tin(II) sulfate is that it loves water! It can pull water right out of the air. When it absorbs enough water, it starts to dissolve in that water, turning into a liquid solution.
How is Tin(II) Sulfate Made?
Scientists can make Tin(II) sulfate in a couple of ways.
- One way is to dissolve pure tin metal in a strong liquid called sulfuric acid.
- Another method involves mixing tin metal with a blue chemical called copper(II) sulfate. When these two react, they create Tin(II) sulfate.
What is Tin(II) Sulfate Used For?
Tin(II) sulfate is very useful when you need a pure source of tin with that specific "+2" charge. Sometimes, tin can also have a "+4" charge, which is called tin(IV). Tin(II) sulfate is important because it provides tin in its "+2" form without being mixed up with the "+4" form. This purity is important for certain chemical reactions or industrial processes.
More About Tin Compounds
 | Jackie Robinson |
 | Jack Johnson |
 | Althea Gibson |
 | Arthur Ashe |
 | Muhammad Ali |