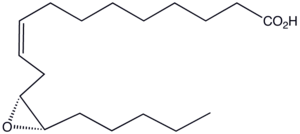
Vernolic acid facts for kids
Vernolic acid is a special type of fatty acid, which is a building block of fats and oils. It's found in some plant oils, especially from plants like Vernonia and Euphorbia. This acid is unique because it has a special ring structure called an epoxide group, which makes it useful for different things.
Where is Vernolic Acid Found?
Vernolic acid is mostly found in a type of oil called vernonia oil. This oil comes from the seeds of the Vernonia galamensis plant, also known as ironweed. This plant grows naturally in eastern Africa.
- The seeds of Vernonia galamensis contain a lot of oil, usually about 40% to 42% of their weight.
- A big part of this oil, about 73% to 80%, is vernolic acid.
- Other plants that contain vernolic acid include Stokesia, Crepis (from the daisy family), and some types of Euphorbia and Bernardia plants.
What Can Vernolic Acid Be Used For?
Vernonia oil, which is rich in vernolic acid, has many possible uses in industry. Because of its unique structure, it can be a good ingredient for making different products.
- It can be used to make adhesives, which are like strong glues.
- It's also useful for creating varnishes and paints.
- Industries can use it for special coatings on surfaces.
- Vernonia oil is not very thick (it has low viscosity). This makes it good for use in oil-based paints. It helps the paint spread easily and becomes part of the dry paint instead of just evaporating.
Vernonia oil is a competitor to other oils like soybean oil or linseed oil that are also used in these industries. Its thinness makes it a good choice compared to other oils that might be thicker.
How Vernolic Acid Affects the Body
In the bodies of animals, including humans, vernolic acid can be made from another common fatty acid called linoleic acid. This happens through natural processes in the body.
- When vernolic acid is produced in the body, it can sometimes affect certain cells, like white blood cells.
- Scientists are studying how vernolic acid might be involved in some health issues, especially when too much of it is present. They are looking into its role in conditions that affect breathing and other body systems.
- It's important to remember that these studies are ongoing, and scientists are still learning about all the ways vernolic acid affects the body.