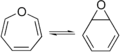Epoxide facts for kids
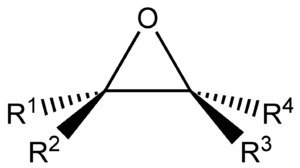
In chemistry, an epoxide is a special kind of molecule. It has a small ring made of three atoms: two carbon atoms and one oxygen atom. These three atoms are connected tightly in a shape like a tiny triangle. Because this ring is so small and tight, epoxides are very eager to react with other chemicals.
Epoxides can be made from other molecules called alkenes. This process is often done using a chemical called a peroxide. Scientists have found many ways to make epoxides. Some methods are so precise that they can even create a specific version of the epoxide molecule, which is very useful for making medicines.
Epoxides love to react with molecules called nucleophiles. When an epoxide meets a nucleophile, its triangular ring usually "pops open." This happens because one of the connections between the oxygen atom and a carbon atom breaks. Once the ring is open, the nucleophile attaches itself to one of the carbon atoms. The oxygen atom then grabs a hydrogen atom, turning into an alcohol. This is very handy because alcohols can easily be changed into many other types of chemical groups.
Epoxides are super useful for making complicated molecules quickly. They are like building blocks that help chemists create many different compounds. This is because the oxygen atom in an epoxide is a great "functional group." Once you have an epoxide, you can easily turn it into other important chemicals like alcohols, ethers, and halohydrins.
Images for kids
See also
 In Spanish: Epóxido para niños
In Spanish: Epóxido para niños
 | Percy Lavon Julian |
 | Katherine Johnson |
 | George Washington Carver |
 | Annie Easley |