Natural rubber facts for kids


Rubber is a stretchy material that comes from nature. It is also known as India rubber or latex. Natural rubber is made of long chains of molecules called polymers. The main ingredient is a compound called isoprene.
Today, most natural rubber comes from the milky, white liquid called latex, which is collected from the Pará rubber tree. This process is called "tapping". The latex is then cleaned and processed to make the rubber we use in everyday products.
Natural rubber is used in thousands of items because it is strong, stretchy, and waterproof. By the late 1800s, people needed more rubber than nature could supply. This led scientists to create synthetic rubber in 1909. Today, countries like Thailand, Indonesia, and Vietnam are the biggest producers of natural rubber.
Where Does Rubber Come From?
The Pará Rubber Tree
The main source of natural rubber is the Pará rubber tree (Hevea brasiliensis). This tree originally grew in the Amazon rainforest in Brazil. Now, it is grown in many tropical parts of the world. Farmers like this tree because it produces a lot of latex for many years when it is cared for properly.
Dandelions
Believe it or not, the white, milky liquid inside a dandelion stem is also a type of latex! This latex has the same quality as the rubber from trees. However, dandelions don't produce very much of it. Scientists are working on ways to grow special dandelions that could one day be used to make rubber on a large scale.
Other Plants
Many other plants also make latex. Some examples include the rubber fig (Ficus elastica) and the Panama rubber tree (Castilla elastica). However, it is often harder to collect and process the latex from these plants than from the Pará rubber tree.
The History of Rubber
Early Uses in the Americas
The first people to use rubber were the native cultures of Central and South America, like the Olmec, Maya, and Aztec. As far back as 1600 BC, they used the latex from rubber trees to make bouncy balls for games. They also used it to make waterproof clothes and containers.
Rubber Arrives in Europe
In the 1700s, European explorers brought rubber back from South America. In 1770, an English scientist named Joseph Priestley discovered that a piece of the material was great for rubbing out pencil marks. This is how it got the name "rubber."
A Stronger Kind of Rubber
Early rubber had problems. It would get sticky when hot and brittle when cold. In 1839, an American inventor named Charles Goodyear discovered a process called vulcanization. By heating rubber with sulfur, he made it much stronger, stretchier, and more durable. This discovery made rubber useful for many more things.
The Rubber Boom
In the 1800s, the demand for rubber grew quickly, especially for tires. South America was the main source of rubber, and it became very valuable. In 1876, an Englishman named Henry Wickham took thousands of rubber tree seeds from Brazil to England. From there, seedlings were sent to parts of Asia, like Malaysia and Sri Lanka. These areas soon became the world's biggest rubber producers.
A Dark Side to the Boom
The high demand for rubber also had a negative side. In some parts of the world, like the Congo in Africa and the Amazon rainforest, workers on rubber plantations were treated very poorly. They were forced to work in harsh conditions to collect as much latex as possible. This part of history shows how the desire for a valuable material can lead to great hardship for people.
What Makes Rubber Special?
Rubber has a special property called elasticity. This means it can be stretched to many times its original length and then spring back to its original shape. Why does it do this? On a tiny, microscopic level, rubber is made of long, tangled chains of molecules. When you stretch rubber, these chains straighten out. When you let go, they curl back up, pulling the rubber back into shape.
When rubber gets very cold, it can become hard and lose its stretchiness. This is because the molecules can't move around as easily. This is what happened to the O-rings on the Space Shuttle Challenger, which led to a tragic accident.
The Science of Rubber
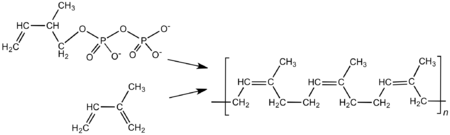
Natural rubber is a polymer. A polymer is a very large molecule made of many smaller, repeating parts linked together in a chain. The small part that repeats in rubber is a molecule called isoprene. So, natural rubber's scientific name is polyisoprene (which means "many isoprenes").
In nature, tiny particles of rubber are made inside special cells in the rubber tree. These particles grow as more isoprene units are added to the chain.
How Is Rubber Made Today?
Today, over 15 million metric tonnes of natural rubber are produced each year. Most of it comes from large farms called plantations in Asia.
Growing Rubber Trees
Rubber trees need a warm, rainy climate to grow well. They are usually grown in huge plantations. A rubber tree can produce latex for about 25 years.
Tapping the Trees
To get the latex, workers make a thin, slanting cut in the tree's bark. This process is called tapping. It doesn't harm the tree if done correctly. The milky white latex then drips out of the cut and is collected in a cup.
Tapping is usually done early in the morning when the tree is full of latex. A skilled worker, called a tapper, can tap hundreds of trees in a few hours. The latex drips for about four hours before it naturally stops.
From Latex to Solid Rubber
The collected latex is taken to a factory. There, an acid (like formic acid) is added to make the liquid latex clump together and become solid. This solid rubber is then washed, pressed into large sheets or blocks, and dried.
The final blocks of rubber are packed up and shipped to factories all over the world to be made into different products.
Making Rubber Stronger: Vulcanization
Natural rubber is useful, but it can be improved. The process of vulcanization makes rubber much stronger, more durable, and more elastic. It also stops the rubber from getting sticky in the heat or brittle in the cold.
During vulcanization, the rubber is heated with sulfur. The sulfur atoms create links between the long polyisoprene chains. These links, called cross-links, hold the chains together, making the rubber tougher. For products like car tires, a black powder called carbon black is also added to make the rubber even stronger and more resistant to wear and tear.
Everyday Uses of Rubber
Rubber is used in thousands of products that we see and use every day. Because it is flexible, waterproof, and durable, it is a very important material.
Some common uses include:
- Tires: The biggest use of rubber is for making tires for cars, trucks, and airplanes.
- Footwear: The soles of many shoes and boots are made of rubber.
- Hoses and Belts: Rubber is used for garden hoses and the belts inside car engines.
- Gloves: Doctors, dentists, and scientists use thin latex gloves to stay safe and clean.
- Household Items: Rubber bands, balloons, and pencil erasers are all made from rubber.
- Waterproof Gear: Raincoats and boots use rubber to keep people dry.
Latex Allergies
Some people have an allergy to the natural proteins found in latex. This is called a latex allergy. If they touch products made from natural rubber, like latex gloves or balloons, they might get a rash or have a more serious reaction.
Because of this, many hospitals now use gloves made from synthetic materials that do not cause allergies. These are often called "latex-free" gloves.
See also
- Akron, Ohio, center of the United States rubber industry
- Crepe rubber
- Ebonite
- Emulsion dispersion
- Fordlândia, failed attempt to establish a rubber plantation in Brazil
- Reinforced rubber
- Resilin, a highly elastic protein found in insects
- Rubber seed oil
- Rubber technology
- Stevenson Plan, historical British plan to stabilize rubber prices
- Charles Greville Williams, researched natural rubber being a polymer of the monomer isoprene
Images for kids
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |