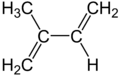Isoprene facts for kids
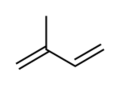
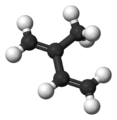
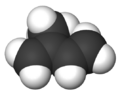
Isoprene is a natural chemical compound that is made by many living things. Its full scientific name is 2-methyl-1,3-butadiene. You can think of it as a building block for other important substances.
Isoprene is a type of hydrocarbon, which means it's made only of carbon and hydrogen atoms. It's also called an unsaturated hydrocarbon because it has double bonds between its carbon atoms. Many plants and animals, including humans, produce isoprene. The main part of natural rubber is made from long chains of isoprene units, called polymers.
Where Isoprene Comes From
Many types of trees release isoprene into the air. Some of the biggest producers are oak trees, poplars, eucalyptus, and certain legumes (plants like peas or beans).
Every year, about 600 million metric tons of isoprene are released by plants. Half of this comes from tropical broadleaf trees (like many flowering plants), and the rest mostly from shrubs. This amount is similar to how much methane is released globally. Isoprene makes up about one-third of all hydrocarbons that go into the atmosphere. In forests where trees lose their leaves in autumn, isoprene can be as much as 80% of the hydrocarbons released. Tiny algae and larger algae also make isoprene, but much less than trees do.
Why Plants Make Isoprene
Plants might make isoprene to help them deal with stress. For example, isoprene can protect plants from moderate heat, like temperatures around 40 °C (104 °F). It can also help plants handle big changes in leaf temperature. Isoprene becomes part of the plant's cell membranes, which are like the skin of the cell. This helps the membranes stay strong and stable.
Isoprene is even responsible for the famous blue mist you see over the Blue Ridge Mountains. This "blue haze" is created when isoprene released by trees reacts with other things in the air.
Images for kids
See also
 In Spanish: Isopreno para niños
In Spanish: Isopreno para niños