Hydrogen sulfate facts for kids
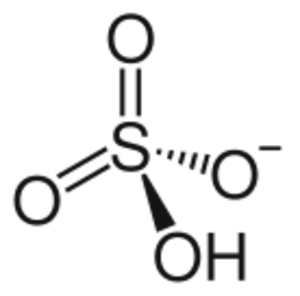
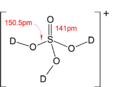
Hydrogen sulfate, also known as bisulfate, is a tiny charged particle called an ion. Its chemical formula is HSO4-. Think of it as a building block that comes from sulfuric acid, which has the formula H2SO4.
What is Hydrogen Sulfate?
Hydrogen sulfate is an ion. This means it's an atom or group of atoms that has an electric charge. The hydrogen sulfate ion has a negative charge. It is formed when sulfuric acid loses one of its hydrogen atoms.
How it's Formed
Sulfuric acid (H2SO4) is a strong acid. When it gives up one of its hydrogen atoms, it becomes the hydrogen sulfate ion (HSO4-). This process is called deprotonation. If sulfuric acid loses both of its hydrogen atoms, it forms a sulfate ion (SO42-).
Where You Find It
Chemical compounds that contain this ion are often called bisulfates or hydrogen sulfates. A common example is sodium bisulfate. These compounds are usually acidic. They can be used as a milder form of acid compared to pure sulfuric acid. You can think of them as a type of salt that comes from sulfuric acid.
Related pages
Images for kids
-
The Rio Tinto is a river with very acidic water.
-
Drain cleaners with sulfuric acid can turn pH paper red and burn it.
-
John Dalton's drawing from 1808 showing a sulfuric acid molecule.