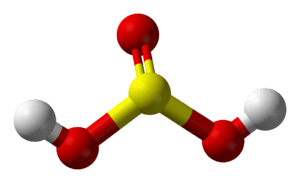
Sulfurous acid facts for kids
Sulfurous acid is a special kind of acid that has the chemical formula H2SO3. Think of a chemical formula as a recipe that tells you exactly which atoms are in a molecule. For sulfurous acid, it means two hydrogen atoms, one sulfur atom, and three oxygen atoms.
This acid is known as a weak acid. This means it doesn't break apart completely when it's mixed with water, unlike strong acids.
You can make sulfurous acid by mixing sulfur dioxide gas with water. It's a bit unstable, meaning it likes to change back into sulfur dioxide and water again.
What is Sulfurous Acid?
Sulfurous acid (H2SO3) is a chemical compound. It's a type of acid that is not very strong. When we say an acid is "weak," it means that when it's in water, only some of its molecules break apart to release hydrogen ions. Strong acids, on the other hand, break apart almost completely.
How is it Formed?
Sulfurous acid is usually formed when sulfur dioxide gas dissolves in water. You might have heard of sulfur dioxide as a gas that comes from volcanoes or from burning fossil fuels. When this gas mixes with water in the air, it can form sulfurous acid. This is part of how acid rain can happen, though sulfuric acid is a bigger part of that.
What Does it Do?
Sulfurous acid can react with other chemicals. For example, bases are chemicals that can react with acids. When a base meets sulfurous acid, it can take away a hydrogen atom from the acid. This process creates new substances called sulfites.
It's also known as a weak reducing agent. In chemistry, a reducing agent is a substance that gives electrons to another substance in a chemical reaction. This causes the other substance to change.
Related pages
See also
 In Spanish: Ácido sulfuroso para niños
In Spanish: Ácido sulfuroso para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |