Chloroform facts for kids
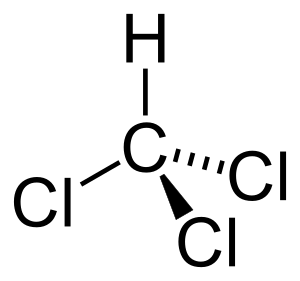
Chloroform (also known as trichloromethane) is a special chemical substance. It is an organic compound. Chloroform is used to make other important chemicals, like Polytetrafluoroethylene, which you might know as Teflon. Teflon is often used for non-stick pans. Chloroform is also a good solvent, meaning it can dissolve other substances. In the 19th century, it was widely used as an anaesthetic to make people sleep during surgery.
Contents
How Chloroform Was Discovered
Chloroform was first made in 1831 by a French chemist named Eugène Soubeiran. He used common chemicals like acetone and ethanol with bleach. Around the same time, an American doctor, Samuel Guthrie, and a German chemist, Justus von Liebig, also made it. In 1834, another French chemist, Jean-Baptiste Dumas, gave it the name "chloroform" and figured out its chemical makeup.
How Chloroform Is Made
Today, chloroform is usually made by mixing methane gas and chlorine gas. This reaction happens at very high temperatures, around 400 to 500 °C. When these two gases mix, they create a blend of different chemicals, including chloroform.
After the reaction, these different chemicals are separated using a process called distillation. This process heats the mixture to different temperatures, allowing each chemical to turn into a gas and then cool back into a liquid at different times.
In laboratories, smaller amounts of chloroform can be made by combining sodium hypochlorite (which is in bleach) and acetone.
What Chloroform Is Used For
Chloroform has several uses, but some have changed over time:
- It is mainly used as a solvent to dissolve other materials.
- It was once used as an anaesthetic to make people unconscious for medical procedures. However, it is no longer used for this because it has many harmful side effects.
- It can act as a catalyst, which means it helps other chemical reactions happen faster without being used up itself.
Why Chloroform Can Be Dangerous
Chloroform was used as an anaesthetic for surgeries and childbirth starting around 1847. It replaced another substance called ether. However, chloroform is very poisonous. It can cause serious breathing problems and issues with the heart. In some cases, it can even cause the heart to stop, which is called cardiac arrest.
If chloroform is stored for a long time, it can break down into a very dangerous gas called Phosgene. Phosgene was used as a poison gas during the First World War.
Chloroform can also cause problems for unborn babies and may lead to miscarriages. There is also a risk that it could cause cancer. Because of these serious risks, chloroform is not used as a medicine anymore.
Images for kids
See also
 In Spanish: Cloroformo para niños
In Spanish: Cloroformo para niños
 | Charles R. Drew |
 | Benjamin Banneker |
 | Jane C. Wright |
 | Roger Arliner Young |