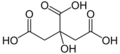
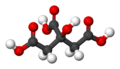
Citric acid facts for kids
Citric acid is a mild organic acid that you can find naturally in citrus fruits. It's often used to keep foods fresh and to give them a sour (acidic) taste. You'll find it in many foods and soft drinks. In the European Union, it's known as E 330 when used as a food additive.
People started making citric acid on a large scale in 1890, using citrus fruits from Italy. Today, more than a million tons of citric acid are made every year!
The first person to successfully get citric acid from lemons was Carl Wilhelm Scheele in 1782. However, some people believe that the alchemist Geber might have discovered it even earlier, in the 9th century, though it might have had a different name back then.
Contents
Where Citric Acid Comes From
Citric acid is found in many fruits and vegetables, but it's most common in citrus fruits. Lemons and limes have a lot of this acid; it can make up as much as 8% of their dry weight!
How We Use Citric Acid
In Food and Drinks

Citric acid is one of the strongest edible acids. Because of this, its main use is to add flavor and act as a preservative in foods and drinks, especially soft drinks.
It can be added to ice cream to help keep fats from separating. It's also used in caramel to stop sugar from forming crystals. Sometimes, people use citric acid in recipes instead of fresh lemon juice. You might also find it mixed with sodium bicarbonate in fizzy products like powders, tablets, bath salts, and bath bombs.
For Cleaning and Binding Metals
Citric acid is great at binding with metals, which helps make them dissolve in water. This is why it's used to remove and prevent limescale buildup in things like boilers. It can also be used to treat water, making soaps and laundry detergents work better. By binding to metals in hard water, citric acid helps cleaners create more foam and clean more effectively without needing to soften the water first.
You'll find citric acid as the main ingredient in some bathroom and kitchen cleaning products. For example, a solution with six percent citric acid can remove hard water stains from glass without scrubbing. In factories, it's used to dissolve rust from steel. Citric acid can even be used in shampoo to wash out wax and coloring from hair.
Safety Information
Even though citric acid is a weak acid, touching the pure form can cause some problems. If you breathe it in, it might cause a cough, shortness of breath, or a sore throat. Eating too much of it can lead to stomach pain and a sore throat. If strong solutions touch your skin or eyes, they can cause redness and pain. Also, using or eating it often over a long time might wear away your tooth enamel.
Images for kids
See also
 In Spanish: Ácido cítrico para niños
In Spanish: Ácido cítrico para niños