Hepatitis B vaccine facts for kids
The Hepatitis B vaccine is a special shot that helps your body fight off the hepatitis B virus. This virus can make you very sick. Doctors usually recommend the first shot within 24 hours of a baby's birth. After that, babies get two or three more doses.
Even people with a weak immune system, like those with HIV/AIDS, or babies born early, should get this vaccine. It's also important for healthcare workers to be vaccinated. For most healthy people, getting all the shots protects over 95% of them from the virus.
If you're at high risk, doctors might do a blood test to check if the vaccine worked. People with weak immune systems might need extra shots. If someone has been near the hepatitis B virus but hasn't been vaccinated, they might get a special medicine called hepatitis B immune globulin along with the vaccine. The vaccine is given as a shot into a muscle.
Serious problems from the hepatitis B vaccine are very rare. You might feel some pain where you got the shot. It's safe for pregnant people and those who are breastfeeding. This vaccine has not been linked to a rare condition called Guillain–Barré syndrome. Hepatitis B vaccines are made using modern recombinant DNA methods. They contain special ingredients that help your immune system learn to fight the virus. You can get this vaccine by itself or combined with other vaccines.
The first hepatitis B vaccine was approved in the United States in 1981. A newer, safer version made with recombinant DNA came out in 1986. This vaccine is on the World Health Organization's List of Essential Medicines, which means it's considered very important for health globally. Both versions were created by a scientist named Maurice Hilleman and his team.
Contents
How the Hepatitis B Vaccine Helps You
The hepatitis B vaccine is important for protecting people from the hepatitis B virus. This virus can cause serious liver problems, including liver cancer.
Protecting Babies and Kids
In the United States, almost all babies are recommended to get the vaccine when they are born. Many countries around the world also give this vaccine to infants. In places where hepatitis B infection is common, vaccinating newborns has greatly reduced the number of infections. It has also led to a big drop in liver cancer in children. For example, in Taiwan, a vaccination program started in 1984 helped lower childhood liver cancer rates.
Protecting Healthcare Workers
Many areas also require healthcare and lab workers to get vaccinated against hepatitis B. This protects them because they might come into contact with the virus.
Special Vaccine Combinations
The World Health Organization (WHO) suggests a "pentavalent vaccine." This single shot combines protection against five diseases: diphtheria, tetanus, pertussis (whooping cough), Haemophilus influenzae type B, and hepatitis B. Another combined vaccine, approved in the U.S., protects against diphtheria, tetanus, pertussis, hepatitis B, and poliomyelitis.
Protecting Babies from Infected Mothers
If a baby is born to a mother who has the hepatitis B virus, the baby can get the virus too. Giving the baby the hepatitis B vaccine, or a special medicine called hepatitis B immunoglobulin, or both, can help prevent the infection. Giving both the vaccine and the immunoglobulin works best to protect these babies.
How Well Does the Vaccine Work?
Studies show that your body remembers how to fight hepatitis B for at least 30 years after you get the vaccine. This memory protects you from getting sick and from long-term hepatitis B infection. This is true even if the levels of protective substances in your blood drop very low.
Most people don't need to be tested to see if the vaccine worked. However, testing is recommended for certain groups. This includes babies born to mothers with hepatitis B, healthcare workers, and people with weakened immune systems. People with weakened immune systems include those on dialney dialysis, HIV patients, or those getting chemotherapy.
A good level of protection is when your blood has more than 100 mIU/ml of a specific antibody. About 85–90% of people reach this level. If your antibody level is between 10 and 100 mIU/ml, you might need one booster shot. If your level is below 10 mIU/ml, you should be checked for past or current infection. Then, you might need another full series of three shots.
Some things can make the vaccine less effective. These include being over 40 years old, being obese, having celiac disease, or smoking. People with weakened immune systems or those on dialysis might need more shots or higher doses.
How Long Does Protection Last?
Scientists now believe that the hepatitis B vaccine gives you protection for a very long time, possibly for life. Older ideas suggested that protection might only last five to seven years. But newer studies show that even after 30 years, your immune system can quickly make antibodies if it sees the virus again. This means your body remembers how to fight the virus, even if antibody levels are low. So, most people who are successfully vaccinated don't need booster shots or retesting.
Possible Side Effects
Serious side effects from the hepatitis B vaccine are very rare. The most common side effect is pain where you get the shot. The vaccine is generally safe for pregnant people and those who are breastfeeding. It has not been linked to Guillain–Barré syndrome, a rare nerve disorder.
Hepatitis B Vaccine and Multiple Sclerosis
Some studies have looked into whether the hepatitis B vaccine is linked to multiple sclerosis (MS) in adults. Most studies have found no connection between the vaccine and MS. One study in 2004 did report a small increase in risk within three years of vaccination, but this study had some problems with how it was done. This discussion caused some people to worry about the vaccine, and vaccination rates in children dropped in some countries. However, a 2006 study concluded that there was no link between the hepatitis B vaccine and sudden infant death syndrome, chronic fatigue syndrome, or multiple sclerosis. A 2007 study also found that the vaccine does not seem to increase the risk of MS in children.
How Many People Get Vaccinated?
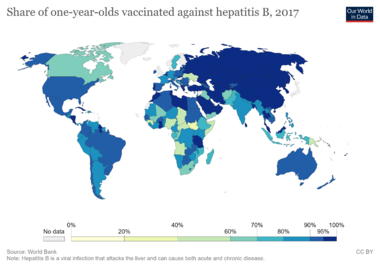
The World Health Organization (WHO) collects information on how many one-year-old children around the world have received all three doses of the hepatitis B vaccine. The table below shows the percentage of vaccinated children in different countries in 2017.
| Hepatitis B (HepB3) immunization coverage among one-year-olds worldwide |
|
|---|---|
| Country | Coverage % |
| Afghanistan | 65 |
| Albania | 99 |
| Algeria | 91 |
| Andorra | 98 |
| Angola | 52 |
| Antigua and Barbuda | 95 |
| Argentina | 86 |
| Armenia | 94 |
| Australia | 95 |
| Austria | 90 |
| Azerbaijan | 95 |
| Bahamas | 94 |
| Bahrain | 98 |
| Bangladesh | 97 |
| Barbados | 90 |
| Belarus | 98 |
| Belgium | 97 |
| Belize | 88 |
| Benin | 82 |
| Bhutan | 98 |
| Bolivia (Plurinational State of) | 83 |
| Bosnia and Herzegovina | 77 |
| Botswana | 95 |
| Brazil | 93 |
| Brunei Darussalam | 99 |
| Bulgaria | 92 |
| Burkina Faso | 91 |
| Burundi | 91 |
| Côte d'Ivoire | 84 |
| Cabo Verde | 86 |
| Cambodia | 93 |
| Cameroon | 86 |
| Canada | 69 |
| Central African Republic | 47 |
| Chad | 41 |
| Chile | 93 |
| China | 99 |
| Colombia | 92 |
| Comoros | 91 |
| Congo | 69 |
| Cook Islands | 99 |
| Costa Rica | 97 |
| Croatia | 94 |
| Cuba | 99 |
| Cyprus | 97 |
| Czech Republic | 94 |
| Democratic People's Republic of Korea | 97 |
| Democratic Republic of the Congo | 81 |
| Djibouti | 68 |
| Dominica | 91 |
| Dominican Republic | 81 |
| Ecuador | 84 |
| Egypt | 94 |
| El Salvador | 85 |
| Equatorial Guinea | 25 |
| Eritrea | 95 |
| Estonia | 92 |
| Eswatini | 90 |
| Ethiopia | 73 |
| Fiji | 99 |
| France | 90 |
| Gabon | 75 |
| Gambia | 92 |
| Georgia | 91 |
| Germany | 87 |
| Ghana | 99 |
| Greece | 96 |
| Grenada | 96 |
| Guatemala | 82 |
| Guinea | 45 |
| Guinea-Bissau | 87 |
| Guyana | 97 |
| Haiti | 58 |
| Honduras | 97 |
| India | 88 |
| Indonesia | 79 |
| Iran (Islamic Republic of) | 99 |
| Iraq | 63 |
| Ireland | 95 |
| Israel | 97 |
| Italy | 94 |
| Jamaica | 93 |
| Jordan | 99 |
| Kazakhstan | 99 |
| Kenya | 82 |
| Kiribati | 90 |
| Kuwait | 99 |
| Kyrgyzstan | 92 |
| Lao People's Democratic Republic | 85 |
| Latvia | 98 |
| Lebanon | 78 |
| Lesotho | 93 |
| Liberia | 86 |
| Libya | 94 |
| Lithuania | 94 |
| Luxembourg | 94 |
| Macedonia | 91 |
| Madagascar | 74 |
| Malawi | 88 |
| Malaysia | 98 |
| Maldives | 99 |
| Mali | 66 |
| Malta | 88 |
| Marshall Islands | 82 |
| Mauritania | 81 |
| Mauritius | 96 |
| Mexico | 93 |
| Micronesia (Federated States of) | 80 |
| Monaco | 99 |
| Mongolia | 99 |
| Montenegro | 73 |
| Morocco | 99 |
| Mozambique | 80 |
| Myanmar | 89 |
| Namibia | 88 |
| Nauru | 87 |
| Nepal | 90 |
| Netherlands | 92 |
| New Zealand | 94 |
| Nicaragua | 98 |
| Niger | 81 |
| Nigeria | 42 |
| Niue | 99 |
| Oman | 99 |
| Pakistan | 75 |
| Palau | 98 |
| Panama | 81 |
| Papua New Guinea | 56 |
| Paraguay | 91 |
| Peru | 83 |
| Philippines | 88 |
| Poland | 95 |
| Portugal | 98 |
| Qatar | 97 |
| Republic of Korea | 98 |
| Republic of Moldova | 89 |
| Romania | 92 |
| Russian Federation | 97 |
| Rwanda | 98 |
| Saint Kitts and Nevis | 98 |
| Saint Lucia | 80 |
| Saint Vincent and the Grenadines | 99 |
| Samoa | 73 |
| San Marino | 86 |
| São Tomé and Príncipe | 95 |
| Saudi Arabia | 98 |
| Senegal | 91 |
| Serbia | 93 |
| Seychelles | 98 |
| Sierra Leone | 90 |
| Singapore | 96 |
| Slovakia | 96 |
| Solomon Islands | 99 |
| Somalia | 42 |
| South Africa | 66 |
| Spain | 93 |
| Sri Lanka | 99 |
| Sudan | 95 |
| Suriname | 81 |
| Swaziland | 98 |
| Sweden | 76 |
| Syrian Arab Republic | 52 |
| Tajikistan | 96 |
| Thailand | 99 |
| Timor-Leste | 76 |
| Togo | 90 |
| Tonga | 81 |
| Trinidad and Tobago | 89 |
| Tunisia | 98 |
| Turkey | 96 |
| Turkmenistan | 99 |
| Tuvalu | 96 |
| Uganda | 85 |
| Ukraine | 52 |
| United Arab Emirates | 98 |
| United Republic of Tanzania | 97 |
| United States of America | 93 |
| Uruguay | 95 |
| Uzbekistan | 99 |
| Vanuatu | 85 |
| Venezuela (Bolivarian Republic of) | 84 |
| Viet Nam | 94 |
| Yemen | 68 |
| Zambia | 94 |
| Zimbabwe | 89 |
History of the Hepatitis B Vaccine
The development of the hepatitis B vaccine is an interesting story of scientific discovery.
Early Discoveries
In 1963, a scientist named Baruch Blumberg found something he called the "Australia Antigen" in the blood of an Australian Aboriginal person. Later, in 1968, another scientist, Alfred Prince, discovered that this protein was actually part of the hepatitis B virus.
In 1976, Blumberg won the Nobel Prize in Physiology and Medicine for his work on hepatitis B. He had found the Australia antigen, which was the first big step. He then figured out how to make the first hepatitis B vaccine. This vaccine was unique because it used parts of the virus taken directly from the blood of people who carried the virus.
The First Vaccine from Blood
Over the next few years, more scientists, including Maurice Hilleman from Merck, worked on the vaccine. In 1980, the first large-scale tests of this blood-derived vaccine were published.
Maurice Hilleman developed a way to clean blood serum using special treatments. This process made a safe vaccine. He believed that injecting people with just the hepatitis B surface protein (a part of the virus) would be safe. This protein doesn't contain the virus's DNA, so it can't cause infection. The idea was that the immune system would learn to recognize and fight these proteins. Then, if the person ever got infected with the real virus, their body would be ready to destroy it quickly.
Hilleman collected blood from groups of people who were at high risk for hepatitis B. He created a multi-step process to purify this blood. This process killed all known viruses, making the vaccine safe. The vaccine was approved in 1981.
The Modern Recombinant Vaccine
The blood-derived hepatitis B vaccine was replaced in 1986 by an improved version. This new vaccine was also developed by Maurice Hilleman and his team at Merck & Co.. It was the first human vaccine ever made using recombinant DNA methods. This means scientists used yeast cells (like those used to make bread) to produce the vaccine.
The recombinant vaccine uses the gene for the hepatitis B surface antigen (HBsAg) inserted into yeast cells. This way, the yeast cells only produce the harmless surface protein, without any risk of getting actual virus DNA into the vaccine. This makes the vaccine very safe. The vaccine also contains a special ingredient called an adjuvant that helps your immune system respond better.
In 2017, a new two-dose hepatitis B vaccine for adults, called Heplisav-B, was approved in the U.S. In November 2021, another new vaccine, Prehevbrio, was also approved by the FDA.
When is the Vaccine Given?
In 1991, the US CDC recommended that all newborns get the hepatitis B vaccine. Before this, it was only recommended for people at high risk. The hepatitis B vaccine is one of the very few vaccines routinely given to babies right after they are born.
How the Vaccine is Made
The hepatitis B vaccine contains a specific protein from the outside of the hepatitis B virus, called Hepatitis B surface antigen (HBsAg). This protein is made by yeast cells. Scientists put the gene for HBsAg into these yeast cells. The yeast then produces the protein, which is collected and used to make the vaccine.
When you get the vaccine, your immune system learns to make an antibody called anti-HBs. This antibody, along with your immune system's memory, helps protect you from getting infected with the hepatitis B virus in the future.
Vaccine Brands
There are several common brands of hepatitis B vaccine available. These include Recombivax HB, Engerix-B, Heplisav-B, and Prehevbrio.
Some vaccines combine hepatitis B protection with other vaccines:
- Twinrix protects against hepatitis A and hepatitis B.
- Pediarix protects against diphtheria, tetanus, pertussis, hepatitis B, and poliomyelitis.
- Vaxelis protects against diphtheria, tetanus, pertussis, poliomyelitis, Haemophilus influenzae type B, and hepatitis B.