Leidenfrost effect facts for kids
Have you ever sprinkled water on a very hot pan and seen the droplets skitter and dance around instead of just sizzling away? This cool phenomenon is called the Leidenfrost effect.
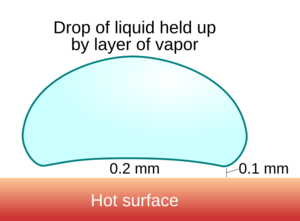
It happens when a liquid touches a surface that is much hotter than the liquid's boiling point. The liquid creates a thin layer of vapor (gas) underneath it. This vapor layer acts like a cushion, lifting the droplet so it hovers just above the hot surface instead of touching it directly. Because of this cushion, the droplet takes much longer to boil and evaporate.
The effect is named after a German doctor, Johann Gottlob Leidenfrost, who wrote about it in 1751.
Contents
How Does the Leidenfrost Effect Work?
You can see this effect easily in the kitchen. Imagine you are heating a pan on the stove and sprinkle a few drops of water on it at different temperatures.
- On a warm pan: If the pan is cooler than the boiling point of water (100 °C (212 °F)), the water just sits there as a liquid and slowly warms up.
- On a hot pan: If the pan is just above boiling temperature, the water will hiss loudly, spread out, and turn into steam very quickly.
- On a super-hot pan: When the pan gets much hotter, past a certain point called the Leidenfrost point (about 193 °C (379 °F) for water), something amazing happens. The water droplets pull together into little balls and glide across the pan as if they were on an air hockey table. They last much longer than they did on the simply hot pan.
This happens because the bottom of the water droplet instantly boils when it gets near the super-hot surface. This creates a pocket of steam that pushes the rest of the droplet up. Since steam doesn't transfer heat very well, this vapor layer protects the liquid water from the intense heat of the pan, making it evaporate much more slowly.
The Leidenfrost Point
The Leidenfrost point is the specific temperature at which the effect starts. It's the temperature where the vapor cushion becomes stable enough to support the liquid droplet.
This temperature isn't always the same. It can change depending on a few things:
- The type of liquid (different liquids have different boiling points).
- The type of surface (a smooth pan might have a different Leidenfrost point than a rough one).
- Any impurities in the liquid.
A Bit of History
While Johann Gottlob Leidenfrost is famous for describing this effect, he wasn't the only one to notice it. In the 1800s, a steam boiler designer named William Fairbairn also studied it. He realized that if the metal in a steam boiler got too hot, it would actually heat the water more slowly because of the Leidenfrost effect. This was an important discovery for making steam engines safer and more efficient.
Cool Examples of the Effect
The Leidenfrost effect isn't just for water in a pan. It shows up in some other surprising ways.
Liquid Nitrogen
The effect also works in reverse with very cold liquids. Liquid nitrogen is extremely cold, with a boiling point of -196°C (-321°F). If a small amount of liquid nitrogen is spilled on a surface at room temperature (like your hand), your skin is so much hotter than the liquid's boiling point that it creates an immediate Leidenfrost effect.
A vapor layer of nitrogen gas forms between your skin and the liquid, protecting you from the extreme cold for a few seconds and allowing the droplets to roll off harmlessly. Warning: This is very dangerous and should never be tried. Scientists and professionals only do this under controlled conditions.
Reactive Leidenfrost Effect
In 2015, scientists discovered that some solid materials can also show a similar effect. When tiny particles of cellulose (the material that makes up plant cell walls) were dropped on a surface hotter than 750 °C (1,380 °F), they began to float and skitter around. This happens because the intense heat breaks down the cellulose, releasing gases that lift the particle up, creating a "reactive Leidenfrost effect."
See also
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |