Manganate facts for kids
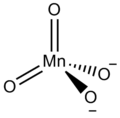
A manganate is a special kind of chemical particle called an ion. It's usually the MnO42- ion, which means it's made of one manganese atom and four oxygen atoms, and it has a negative electrical charge. Manganate ions are known for their bright green color when dissolved in water.
These green ions are often formed when people are making other important chemicals called permanganates. Think of it like a step in a recipe! Manganate ions can react with other chemicals, sometimes giving away parts of themselves and sometimes taking parts from others. This makes them useful in different chemical processes. They are quite similar to other common ions you might hear about, like sulfate (SO42-) or chromate (CrO42-). A good example of a manganate is potassium manganate.
What is a Manganate?
A manganate is a chemical compound that contains the manganate ion. This ion is made up of a central manganese atom surrounded by four oxygen atoms. The "2-" in its formula (MnO42-) tells us it has a negative charge, which is why it's called an ion. Ions are atoms or molecules that have gained or lost electrons, giving them an electrical charge.
How Manganates are Made
Manganates are often created as an intermediate step when making permanganates. Permanganates are another type of manganese compound, but they have a different charge and are usually purple. For example, when you heat manganese dioxide with a strong base like potassium hydroxide in the presence of air, you can form potassium manganate. This process shows how manganese can change its chemical form depending on the conditions.
What Manganates Do
Manganate ions can act as both a weak reducing agent and a moderate oxidizing agent. This means they can either donate electrons to other chemicals (acting as a reducing agent) or accept electrons from other chemicals (acting as an oxidizing agent). This ability to swap electrons makes them useful in various chemical reactions, though they are not as strong as their purple cousins, the permanganates.
Images for kids
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |