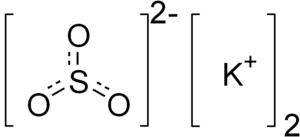Potassium sulfite facts for kids
Potassium sulfite is a chemical compound. Think of it as a tiny building block made of different parts. Its chemical formula is K2SO3. This means it has two potassium atoms, one sulfur atom, and three oxygen atoms all joined together. It contains potassium and sulfite ions, which are like tiny charged particles.
Contents
What Is Potassium Sulfite?
Potassium sulfite is a special kind of chemical. It's made up of potassium and a group of atoms called sulfite. These two parts come together to form this compound. It's often found as a white, solid material.
How It Looks and Acts
Potassium sulfite is a white, crystalline solid. This means it looks like tiny, shiny crystals, similar to salt or sugar. It is also known as a reducing agent. This means it can easily give away some of its electrons in chemical reactions. When it meets an oxidizing agent, which likes to take electrons, they react. This reaction can turn potassium sulfite into potassium sulfate.
How It's Made
Scientists and chemists can make potassium sulfite. They do this by mixing two other chemicals. They react sulfur dioxide gas with potassium hydroxide. Potassium hydroxide is a strong base. When these two chemicals meet, they create potassium sulfite and water.
Here's the chemical recipe:
- 2 KOH + SO2 → K2SO3 + H2O
This means two parts of potassium hydroxide plus one part of sulfur dioxide make one part of potassium sulfite and one part of water.
What It's Used For
One of the main jobs for potassium sulfite is to preserve food. It helps keep food fresh for longer periods. It can stop food from spoiling or changing color. You might find it in some dried fruits or wines. However, it's important to know that sulfites can cause allergies in some people. If someone has a sulfite allergy, they need to avoid foods that contain them.
Related Pages
See also
 In Spanish: Sulfito de potasio para niños
In Spanish: Sulfito de potasio para niños
 | Janet Taylor Pickett |
 | Synthia Saint James |
 | Howardena Pindell |
 | Faith Ringgold |