Structural isomer facts for kids
A structural isomer (also called a constitutional isomer) is a type of compound that has the exact same number of atoms of each element as another compound, but these atoms are connected in a different way. Think of it like having the same set of LEGO bricks, but building two completely different models with them!
Structural isomers are different from stereoisomerism, where the atoms are connected in the same order but are arranged differently in 3D space.
There are three main types of structural isomers:
- Skeletal isomers (also called chain isomers)
- Positional isomers (also called regioisomers)
- Functional isomers
Contents
Different Shapes of the Carbon Chain
In skeletal isomerism, also known as chain isomerism, the main "backbone" of the molecule (usually made of carbon atoms) is arranged differently. Imagine a chain of beads: you can arrange them in a straight line, or make them branch off.
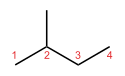
For example, the chemical compound pentane has three different skeletal isomers. All three have 5 carbon atoms and 12 hydrogen atoms (C5H12), but their carbon chains look different:
- n-pentane (which is often just called "pentane") has a straight chain.
- Isopentane (also called methylbutane) has one branch.
- Neopentane (also called dimethylpropane) has two branches, making it more compact.
 |
 |
|
| n-Pentane | Isopentane | Neopentane |
Changing Where Things Attach
In position isomerism, a special group of atoms called a functional group or another part of the molecule moves to a different spot on the main structure. The main carbon chain stays the same, but where something is attached changes.
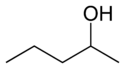
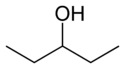
For example, consider an alcohol molecule with a 5-carbon chain. The "hydroxyl group" (which is an oxygen atom connected to a hydrogen atom, -OH) can be attached to different carbon atoms along the chain. This creates different compounds, even though they have the same number of atoms.
 |
 |
|
| 1-Pentanol | 2-Pentanol | 3-Pentanol |
Another good example is with aromatic compounds, which have ring-shaped structures. If you have a benzene ring with other atoms or groups attached, changing their positions on the ring can create different isomers. For instance, cresol has a hydroxyl group and a methyl group on a benzene ring. Depending on where the methyl group is placed, there are three different cresol isomers.
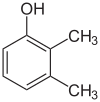
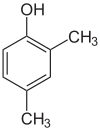
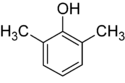
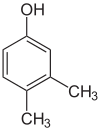
Xylenol is another example. It has one hydroxyl group and two methyl groups attached to a benzene ring. Because of the different ways these groups can be placed, there are 6 different xylenol isomers!
 |
 |
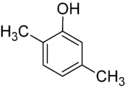 |
| 2,3-Xylenol | 2,4-Xylenol | 2,5-Xylenol |
 |
 |
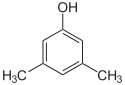 |
| 2,6-Xylenol | 3,4-Xylenol | 3,5-xylenol |
Different Chemical Groups
Functional isomers are structural isomers that have the same molecular formula (meaning they have the exact same number of each type of atom), but these atoms are connected in ways that create different functional groups. Functional groups are specific groups of atoms within a molecule that give it certain chemical properties.
So, if two compounds have the same "ingredients" (molecular formula) but are "built" with different main chemical "parts" (functional groups), they are functional isomers.
For example, both cyclohexane and 1-hexene have the molecular formula C6H12. However, they are very different compounds:
- Cyclohexane is a cycloalkane, which means it's a ring of carbon atoms with only single bonds.
- 1-hexene is an alkene, which means it's a straight chain of carbon atoms with at least one double bond.
Because they have different types of carbon-carbon bonds and structures (a ring vs. a chain with a double bond), they are functional isomers.
 |
|
| Cyclohexane | 1-hexene |
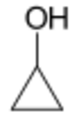
Another common example is with the molecular formula C2H6O. This formula can represent two different compounds:
- Dimethyl ether (CH3-O-CH3)
- Ethanol (CH3CH2-O-H)
Dimethyl ether and ethanol are functional isomers. Dimethyl ether is an ether, which means it has an oxygen atom connected to two carbon chains (carbon-oxygen-carbon). Ethanol is an alcohol, which means it has an oxygen atom connected to a carbon chain and a hydrogen atom (carbon-oxygen-hydrogen). These are two different functional groups, even though the total number of atoms is the same for both molecules.
It's important to remember that not all structural isomers are functional isomers. For instance, 1-propanol and 2-propanol are structural isomers (because the -OH group is in a different position), but they are both alcohols. They have the same functional group (alcohol), just in different places. So, they are not functional isomers.
Chemists often use a technique called infrared spectroscopy to identify functional isomers. Different functional groups absorb infrared light at specific wavelengths, which creates a unique "fingerprint" that helps identify the type of molecule. For example, alcohols have a very distinct signal in their infrared spectrum because of the way their O-H bond vibrates.
In short, functional isomers are structural isomers that have completely different types of chemical groups or "parts" within their structure.
Images for kids