VRLA battery facts for kids

A valve-regulated lead-acid (VRLA) battery is a special kind of lead–acid battery. It's often called a sealed lead-acid (SLA) battery. These batteries have their liquid (called electrolyte) held in place. It's either soaked into a mat or turned into a gel. They also have a special valve that lets out gas if the pressure gets too high. This design means the battery can work in any position.
There are two main types of VRLA batteries:
- Absorbent glass mat (AGM) batteries use a fiberglass mesh to hold the electrolyte.
- Gel cell (gel battery) batteries mix silica dust into the electrolyte. This makes it a thick, jelly-like substance.
Both AGM and gel batteries are different from older, "flooded" lead-acid batteries. They don't need much looking after. You can use them in many places where you need a lot of power storage. They are also cheaper than some newer battery types like lithium-ion batteries.
Contents
How VRLA Batteries Started
The very first lead-acid gel battery was made in Germany in 1934. The modern gel or VRLA battery was invented by Otto Jache in 1957. The first AGM battery was called the Cyclon. It was patented in 1972. Later, in the 1980s, companies in the UK started making larger AGM batteries. These were used for things like new digital phone systems.
How These Batteries Work
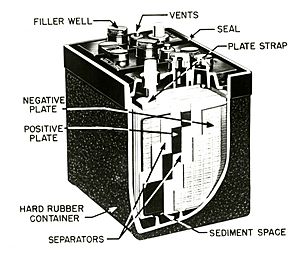
Lead-acid batteries have two lead plates. These plates are in a liquid called sulfuric acid. When the battery gives out power, the lead and acid change. They create lead sulfate and water. When you charge the battery, this process reverses. The lead sulfate and water turn back into lead and acid.
In VRLA batteries, the liquid acid is held still. In AGM batteries, it's in a fiberglass mat. In gel batteries, it's a thick, jelly-like paste.
When you charge a battery, it's important not to charge it too fast. If you do, water can break down into hydrogen and oxygen gases. In older batteries, these gases would escape. You would need to add water sometimes. VRLA batteries are different. They keep these gases inside. The gases can then combine back into water. This means you usually don't need to add water. If too much gas builds up, a safety valve opens. This lets out the extra gas and keeps the battery safe. That's why they are called "valve-regulated."
How They Are Built
Each part (cell) of a VRLA battery has a special valve. This valve opens if too much gas builds up inside. This usually happens when the battery is being charged.
The battery covers often have parts that help spread out any extra hydrogen gas. These batteries are not fully sealed forever. But they are made so you don't have to do much to them. You can place them in any direction. Regular lead-acid batteries must stay upright. This is to stop acid from spilling.
Absorbent Glass Mat (AGM) Batteries
AGM batteries are different because their liquid (electrolyte) is held in glass mats. These mats are made of very thin glass fibers. They are woven together to hold enough electrolyte for the battery's life. The glass fibers don't soak up the acid. They are just there to hold it in place.
AGM batteries can have plates of different shapes. Some are flat, while others are bent or rolled up. Both types of AGM batteries (for deep power or starting engines) fit into standard rectangular cases.
AGM batteries don't lose their charge as quickly as regular batteries. This is true across many temperatures. To make an AGM battery last a long time, always follow the maker's charging rules. Using a special charger that controls the voltage is a good idea. How much you use the battery before recharging affects its life. The less you drain it, the longer it will last.
Gel Batteries
An early type of gel battery was made in the 1930s for portable radios. It used silica added to sulfuric acid.
A modern gel battery is a VRLA battery with a gel-like liquid. The sulfuric acid is mixed with a special powder called fumed silica. This makes the liquid thick and still. Unlike older "wet" batteries, gel batteries don't need to be kept upright. Gel batteries also lose less liquid through evaporation. They are less likely to spill acid. This means fewer corrosion problems. They can also handle bumps and shakes better.
Where VRLA Batteries Are Used
Many modern motorcycles and all-terrain vehicles (ATVs) use AGM batteries. This helps stop acid from spilling if the vehicle tips or crashes. It also lets designers put the battery in different places. Because they cost more to make, AGM batteries are often found in fancy cars. As cars get heavier and have more electronics, AGM batteries are used to make them lighter. They also give more reliable power.
Some BMW cars use AGM batteries with a system that recharges the battery when the car slows down. Race cars often use AGM batteries because they can handle a lot of vibration. Old cars also use them because they are less likely to leak acid. This protects hard-to-replace parts.
AGM batteries that can give power for a long time (deep-cycle) are used in off-grid solar power and wind power systems. They store energy. They are also popular in large robotics competitions.
VRLA batteries are great for remote sensors, like those that check ice in the Arctic. Since they don't have free liquid, they won't crack and leak in the cold.
These batteries are also used a lot in power wheelchairs and mobility scooters. They don't let out much gas or acid. This makes them safer for use indoors. VRLA batteries are also used in uninterruptible power supply (UPS) systems. These give backup power when the electricity goes out.
Sailplanes (gliders) use VRLA batteries too. They work well even when the plane is at different angles or in changing temperatures. The US Nuclear Submarine fleet uses them because they are powerful, don't release gas, and are safer.
AGM and gel batteries are also used for boats. AGM marine batteries are common. People like them because they need little care and don't spill.
VRLA vs. Older Batteries
VRLA gel and AGM batteries have several good points compared to older, "flooded" lead-acid batteries:
- You can put the battery in any position. The valves only open if there's too much pressure.
- They don't let out much gas. This means you don't need as much room ventilation. No acid fumes come out during normal use.
- If the case gets damaged, very little liquid can spill out.
- You don't need to check the liquid level or add water. This means less checking and care.
However, all lead-acid batteries take a while to recharge fully. They need a special three-step charging process. If a charger doesn't do this right, the battery won't last as long. To make them last, lead-acid batteries should be recharged soon after use. They should also be kept fully charged when not in use.
It's best not to drain a lead-acid battery too much. Try to keep it from going below 50% empty. A special "deep-cycle" battery can go lower, but it will shorten its life. With good care, lead-acid batteries can last for 500 to 1000 cycles (times they are used and recharged). If not cared for well, they might only last 100 cycles.
Because of special materials added to their plates, sealed AGM or gel batteries recharge faster than older flooded batteries. But VRLA batteries can be damaged more easily by charging them too much. Also, you can't easily check their health with a special tool (hydrometer).
AGM car batteries usually cost about twice as much as flooded batteries. Gel batteries can cost up to five times more.
Here's a quick summary of AGM and gel VRLA batteries:
- They recharge faster than flooded lead-acid batteries.
- Charging them too much will make them fail early.
- They usually don't last as long as well-cared-for older batteries.
- They release much less hydrogen gas.
- AGM batteries are safer for the environment and to use.
- You can use them in any position.
See also
 In Spanish: Batería VRLA para niños
In Spanish: Batería VRLA para niños
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |


