Zinc nitrate facts for kids
Quick facts for kids Zinc nitrate |
|
|---|---|
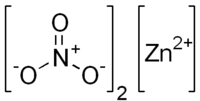 |
|
 |
|
| IUPAC name | Zinc nitrate |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colorless, deliquescent crystals |
| Melting point | |
| Boiling point | |
| Hazards | |
| Main hazards | Oxidant, may explode on heating |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Zinc sulfate Zinc chloride |
| Other cations | Cadmium nitrate Mercury(II) nitrate |
| Related compounds | Copper(II) nitrate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Zinc nitrate is a chemical compound. Its chemical formula is Zn(NO3)2. This means it is made of zinc and nitrate ions. It is often found as a colorless solid.
Contents
What is Zinc Nitrate?
Zinc nitrate is a type of salt. It is made from the metal zinc and a chemical group called nitrate. It looks like clear crystals. These crystals can absorb moisture from the air, which means they are deliquescent.
How is it Made?
You can make zinc nitrate by mixing zinc metal or zinc oxide with nitric acid. Nitric acid is a strong acid. When these chemicals react, they form zinc nitrate and water.
What is it Used For?
Zinc nitrate has a few important uses:
- It can be used as a mordant. In dyeing, a mordant helps the dye stick to the fabric.
- It is also a way to get zinc ions. Zinc ions are important in many chemical reactions.
Safety Information
Zinc nitrate is an oxidizer. This means it can make other substances burn more easily. It can also be dangerous if heated too much. It might even explode if it gets very hot. However, zinc nitrate itself does not catch fire easily.
Related Chemicals
Images for kids
See also
 In Spanish: Nitrato de cinc para niños
In Spanish: Nitrato de cinc para niños


