Absorption spectroscopy facts for kids
Absorption spectroscopy is a cool way to figure out what a substance is made of. Think of it like a detective tool for chemistry!
Imagine shining a full spectrum of light (like sunlight with all its colors) through a sample, often a gas. When the light comes out the other side, some specific colors might be missing. These missing colors were absorbed by the sample. This creates a picture of the light spectrum with dark lines or breaks where the light was absorbed. These dark lines are called absorption lines. Every chemical element has its own unique pattern of these lines, like a fingerprint!
Contents
How Light Gets Absorbed
This happens because of tiny particles called electrons inside the atoms of the sample. An electron can absorb light to gain energy. Scientists have found that electrons only absorb certain amounts of energy. It's like they can only jump to specific "energy levels," not just any level. When an electron jumps to a higher energy level, it's called excitation.
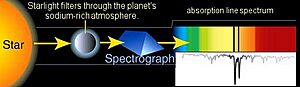
For any atom of a specific element, the energy needed to make an electron jump from one energy level to another is always the same. This is super important! It means we can compare the absorption lines from, say, the atmosphere of a faraway planet to the lines of elements we know here on Earth. This helps us figure out what that distant planet's atmosphere might be made of.
The missing colors tell us about the energy of the light particles, called photons, that caused the electrons to jump. Only photons with the right amount of energy will cause an electron to get excited. The energy of a photon is linked to its color (or frequency). The more energy a photon has, the higher its frequency. This is why those dark breaks in the spectrum help us identify the element(s) that the light passed through.
The amount of light absorbed changes depending on the color (or frequency) of the light. This changing pattern of absorption is called the absorption spectrum.
Using Absorption Spectroscopy in Chemistry
In chemistry, this method helps scientists find and measure how much of a certain metal element is in a solution (like sugar dissolved in water). Scientists first "atomize" the sample, which means they turn it into individual atoms. Then, they shine light through it to see which light wavelengths (colors) it absorbs.
Since each type of chemical (element) absorbs a specific wavelength, scientists can tell exactly which chemicals are in the sample. Every element has a different atomic absorption spectrum because of the unique light wavelengths it absorbs.
Images for kids
-
The infrared absorption spectrum of NASA laboratory sulfur dioxide ice is compared with the infrared absorption spectra of ices on Jupiter's moon, Io credit NASA, Bernard Schmitt, and UKIRT.
-
Absorption spectrum observed by the Hubble Space Telescope
See also
 In Spanish: Espectroscopia de absorción para niños
In Spanish: Espectroscopia de absorción para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |



